The Tumor Immune Profile of Murine Ovarian Cancer Models: An Essential Tool For Ovarian Cancer Immunotherapy Research
- PMID: 36311166
- PMCID: PMC9616009
- DOI: 10.1158/2767-9764.crc-22-0017
The Tumor Immune Profile of Murine Ovarian Cancer Models: An Essential Tool For Ovarian Cancer Immunotherapy Research
Abstract
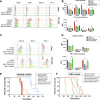
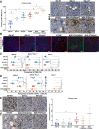
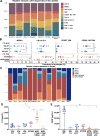
Epithelial ovarian cancer (EOC) is the most lethal gynecologic cancer with an imperative need for new treatments. Immunotherapy has had marked success in some cancer types; however, clinical trials studying the efficacy of immune checkpoint inhibitors for the treatment of EOC benefited less than 15% of patients. Given that EOC develops from multiple tissues in the reproductive system and metastasizes widely throughout the peritoneal cavity, responses to immunotherapy are likely hindered by heterogeneous tumor microenvironments (TME) containing a variety of immune profiles. To fully characterize and compare syngeneic model systems that may reflect this diversity, we determined the immunogenicity of six ovarian tumor models in vivo, the T and myeloid profile of orthotopic tumors and the immune composition and cytokine profile of ascites, by single-cell RNA sequencing, flow cytometry and IHC. The selected models reflect the different cellular origins of EOC (ovarian and fallopian tube epithelium) and harbor mutations relevant to human disease, including Tp53 mutation, PTEN suppression, and constitutive KRAS activation. ID8-p53-/- and ID8-C3 tumors were most highly infiltrated by T cells, whereas STOSE and MOE-PTEN/KRAS tumors were primarily infiltrated by tumor associated macrophages and were unique in MHC class I and II expression. MOE-PTEN/KRAS tumors were capable of forming T cell clusters. This panel of well-defined murine EOC models reflects some of the heterogeneity found in human disease and can serve as a valuable resource for studies that aim to test immunotherapies, explore the mechanisms of immune response to therapy, and guide selection of treatments for patient populations.
Keywords: cold versus hot tumors; immunogenicity; ovarian cancer; tumor infiltrating immune cells; tumor mutational burden.
Conflict of interest statement
Conflicts of Interest The authors declare no potential conflicts of interest.
Figures




Similar articles
-
Cancer cell genotype associated tumor immune microenvironment exhibits differential response to therapeutic STING pathway activation in high-grade serous ovarian cancer.J Immunother Cancer. 2023 Apr;11(4):e006170. doi: 10.1136/jitc-2022-006170. J Immunother Cancer. 2023. PMID: 37015760 Free PMC article.
-
Modification of the Tumor Microenvironment in KRAS or c-MYC-Induced Ovarian Cancer-Associated Peritonitis.PLoS One. 2016 Aug 2;11(8):e0160330. doi: 10.1371/journal.pone.0160330. eCollection 2016. PLoS One. 2016. PMID: 27483433 Free PMC article.
-
Targeting myeloid cells in the tumor microenvironment enhances vaccine efficacy in murine epithelial ovarian cancer.Oncotarget. 2015 May 10;6(13):11310-26. doi: 10.18632/oncotarget.3597. Oncotarget. 2015. PMID: 25888637 Free PMC article.
-
The Transcoelomic Ecosystem and Epithelial Ovarian Cancer Dissemination.Front Endocrinol (Lausanne). 2022 Apr 28;13:886533. doi: 10.3389/fendo.2022.886533. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35574025 Free PMC article. Review.
-
Basics of immunotherapy for epithelial ovarian cancer.J Gynecol Obstet Hum Reprod. 2022 Feb;51(2):102283. doi: 10.1016/j.jogoh.2021.102283. Epub 2021 Dec 5. J Gynecol Obstet Hum Reprod. 2022. PMID: 34875397 Review.
Cited by
-
NLRC5 overexpression in ovarian tumors remodels the tumor microenvironment and increases T-cell reactivity toward autologous tumor-associated antigens.Front Immunol. 2024 Jan 3;14:1295208. doi: 10.3389/fimmu.2023.1295208. eCollection 2023. Front Immunol. 2024. PMID: 38235131 Free PMC article.
-
BRCA1 and BRCA2 deficient tumour models generate distinct ovarian tumour microenvironments and differential responses to therapy.J Ovarian Res. 2023 Nov 28;16(1):231. doi: 10.1186/s13048-023-01313-z. J Ovarian Res. 2023. PMID: 38017453 Free PMC article.
-
BRCA Status Dictates Wnt Responsiveness in Epithelial Ovarian Cancer.Cancer Res Commun. 2024 Aug 1;4(8):2075-2088. doi: 10.1158/2767-9764.CRC-24-0111. Cancer Res Commun. 2024. PMID: 39028933 Free PMC article.
-
Oncogenic Pathways and Targeted Therapies in Ovarian Cancer.Biomolecules. 2024 May 15;14(5):585. doi: 10.3390/biom14050585. Biomolecules. 2024. PMID: 38785992 Free PMC article. Review.
-
Biology-driven therapy advances in high-grade serous ovarian cancer.J Clin Invest. 2024 Jan 2;134(1):e174013. doi: 10.1172/JCI174013. J Clin Invest. 2024. PMID: 38165032 Free PMC article. Review.
References
-
- American Cancer Society. Cancer Facts & Figures; 2021 | American Cancer Society [Internet]. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts...
-
- Berek JS, Hacker NF. Berek and Hacker's gynecologic oncology. 6th ed.Philadelphia, PA: Lippincott Williams & Wilkins; 2015.
-
- Santoiemma PP, Reyes C, Wang L-P, Mclane MW, Feldman MD, Tanyi JL, et al. . Systematic evaluation of multiple immune markers reveals prognostic factors in ovarian cancer. Gynecol Oncol 2016;143:120–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

