Breakthrough of extracellular vesicles in pathogenesis, diagnosis and treatment of osteoarthritis
- PMID: 36311050
- PMCID: PMC9588998
- DOI: 10.1016/j.bioactmat.2022.10.012
Breakthrough of extracellular vesicles in pathogenesis, diagnosis and treatment of osteoarthritis
Abstract
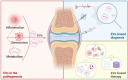
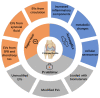
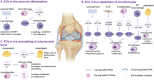
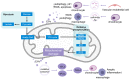
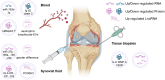
Osteoarthritis (OA) is a highly prevalent whole-joint disease that causes disability and pain and affects a patient's quality of life. However, currently, there is a lack of effective early diagnosis and treatment. Although stem cells can promote cartilage repair and treat OA, problems such as immune rejection and tumorigenicity persist. Extracellular vesicles (EVs) can transmit genetic information from donor cells and mediate intercellular communication, which is considered a functional paracrine factor of stem cells. Increasing evidences suggest that EVs may play an essential and complex role in the pathogenesis, diagnosis, and treatment of OA. Here, we introduced the role of EVs in OA progression by influencing inflammation, metabolism, and aging. Next, we discussed EVs from the blood, synovial fluid, and joint-related cells for diagnosis. Moreover, we outlined the potential of modified and unmodified EVs and their combination with biomaterials for OA therapy. Finally, we discuss the deficiencies and put forward the prospects and challenges related to the application of EVs in the field of OA.
Keywords: Biomarkers; Diagnosis; Extracellular vesicles; Osteoarthritis; Treatment.
© 2022 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Harnessing Tissue-derived Extracellular Vesicles for Osteoarthritis Theranostics.Theranostics. 2022 Jan 1;12(1):207-231. doi: 10.7150/thno.62708. eCollection 2022. Theranostics. 2022. PMID: 34987642 Free PMC article. Review.
-
The role of extracellular vesicles in osteoarthritis treatment via microenvironment regulation.Biomater Res. 2022 Oct 5;26(1):52. doi: 10.1186/s40824-022-00300-7. Biomater Res. 2022. PMID: 36199125 Free PMC article. Review.
-
Plasma-derived extracellular vesicle surface markers CD45, CD326 and CD56 correlate with the stage of osteoarthritis: a primary study of a novel and promising diagnostic tool of the disease.Sci Rep. 2023 Nov 16;13(1):20071. doi: 10.1038/s41598-023-47074-z. Sci Rep. 2023. PMID: 37973964 Free PMC article.
-
Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages.J Nanobiotechnology. 2022 Jan 20;20(1):38. doi: 10.1186/s12951-021-01236-1. J Nanobiotechnology. 2022. PMID: 35057811 Free PMC article.
-
Extracellular Vesicles and Their Potential Significance in the Pathogenesis and Treatment of Osteoarthritis.Pharmaceuticals (Basel). 2021 Apr 1;14(4):315. doi: 10.3390/ph14040315. Pharmaceuticals (Basel). 2021. PMID: 33915903 Free PMC article. Review.
Cited by
-
The Use of Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles in the Treatment of Osteoarthritis: Insights from Preclinical Studies.Bioengineering (Basel). 2024 Sep 26;11(10):961. doi: 10.3390/bioengineering11100961. Bioengineering (Basel). 2024. PMID: 39451337 Free PMC article.
-
The Role of Extracellular Vesicles in Bone Regeneration and Associated Bone Diseases.Curr Issues Mol Biol. 2024 Aug 23;46(9):9269-9285. doi: 10.3390/cimb46090548. Curr Issues Mol Biol. 2024. PMID: 39329900 Free PMC article. Review.
-
Engineering exosomes derived from TNF-α preconditioned IPFP-MSCs enhance both yield and therapeutic efficacy for osteoarthritis.J Nanobiotechnology. 2024 Sep 11;22(1):555. doi: 10.1186/s12951-024-02795-9. J Nanobiotechnology. 2024. PMID: 39261846 Free PMC article.
-
Bibliometric analysis of research on osteoarthritis and extracellular vesicles: Trends and frontiers.Heliyon. 2024 Aug 10;10(16):e36127. doi: 10.1016/j.heliyon.2024.e36127. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39224260 Free PMC article.
-
Stem cell recruitment polypeptide hydrogel microcarriers with exosome delivery for osteoarthritis treatment.J Nanobiotechnology. 2024 Aug 27;22(1):512. doi: 10.1186/s12951-024-02765-1. J Nanobiotechnology. 2024. PMID: 39192268 Free PMC article.
References
-
- Hunter D.J., Schofield D., Callander E. The individual and socioeconomic impact of osteoarthritis, Nature reviews. Rheumatology. 2014;10(7):437–441. - PubMed
-
- Hunter D.J., Bierma-Zeinstra S. Osteoarthritis, Lancet. 2019;393(10182):1745–1759. - PubMed
-
- Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., Goldring S.R., Jones G., Teichtahl A.J., Pelletier J.-P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016;2 - PubMed
Publication types
LinkOut - more resources
Full Text Sources

