Thromboinflammation in Sepsis and Heparin: A Review of Literature and Pathophysiology
- PMID: 36309378
- PMCID: PMC9677778
- DOI: 10.21873/invivo.12991
Thromboinflammation in Sepsis and Heparin: A Review of Literature and Pathophysiology
Abstract
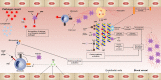
Background/aim: Thromboinflammation is the pathophysiologic mechanism in which coagulation and inflammation interact and complement each other. It is observed in a number of degenerative diseases, one of them being sepsis. Quiescent endothelial cells exert antithrombotic and anti-inflammatory actions that are reduced during sepsis. The concomitant effect of the subsequent dysregulation of coagulation and complement actuation is platelet activation and aggregation, and leukocyte recruitment, with detrimental effects on the vascular endothelium. Tissue factor and α-thrombin are major sentinels in the pathogenesis of this process. This literature review aimed to cover the basic principles of the mechanisms implicated in thromboinflammation occurring during sepsis and also investigates the role of heparin as a possible therapeutic agent, since it exhibits both anticoagulant and anti-inflammatory functions.
Materials and methods: PubMed, SCOPUS and ScienceDirect databases were used for search of literature from inception to April 2022. To be included in our review, studies had to refer to the pathophysiologic mechanisms leading to coincident coagulation and inflammation, or to the administration of heparin either for treatment or prophylaxis, both in the context of sepsis.
Results: A total of 276 articles were drawn from the initial literature search. 124 were duplicated and out of the remaining 152 articles, 29 met our inclusion criteria and were reviewed.
Conclusion: Clinical trials among sepsis patients have indicated that the thromboinflammatory process is more complex than believed, as adverse bleeding events continue to occur despite the use of anticoagulants with different pharmacodynamics. However, heparin has a pleiotropic effect that might provide protection against sepsis and related complications and merits further research.
Keywords: Thromboinflammation; heparin action; immunothrombosis; review; sepsis pathophysiology.
Copyright © 2022, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms.Blood. 2019 Feb 28;133(9):906-918. doi: 10.1182/blood-2018-11-882993. Epub 2019 Jan 14. Blood. 2019. PMID: 30642917 Review.
-
Thromboinflammation in acute injury: infections, heatstroke, and trauma.J Thromb Haemost. 2024 Jan;22(1):7-22. doi: 10.1016/j.jtha.2023.07.020. Epub 2023 Aug 3. J Thromb Haemost. 2024. PMID: 37541590 Review.
-
Heparinization of cell surfaces with short peptide-conjugated PEG-lipid regulates thromboinflammation in transplantation of human MSCs and hepatocytes.Acta Biomater. 2016 Apr 15;35:194-205. doi: 10.1016/j.actbio.2016.02.018. Epub 2016 Feb 12. Acta Biomater. 2016. PMID: 26876877
-
Scorch processing of rhubarb (Rheum tanguticum Maxim. ex Balf.) pyrolyzed anthraquinone glucosides into aglycones and improved the therapeutic effects on thromboinflammation via regulating the complement and coagulation cascades pathway.J Ethnopharmacol. 2024 Oct 28;333:118475. doi: 10.1016/j.jep.2024.118475. Epub 2024 Jun 20. J Ethnopharmacol. 2024. PMID: 38908496
-
Heparin-Functionalized Adsorbents Eliminate Central Effectors of Immunothrombosis, including Platelet Factor 4, High-Mobility Group Box 1 Protein and Histones.Int J Mol Sci. 2022 Feb 5;23(3):1823. doi: 10.3390/ijms23031823. Int J Mol Sci. 2022. PMID: 35163743 Free PMC article.
Cited by
-
Heparin, Heparan Sulphate and Sepsis: Potential New Options for Treatment.Pharmaceuticals (Basel). 2023 Feb 10;16(2):271. doi: 10.3390/ph16020271. Pharmaceuticals (Basel). 2023. PMID: 37259415 Free PMC article. Review.
-
Circulating microRNAs targeting coagulation and fibrinolysis in patients with severe COVID-19.Thromb J. 2024 Sep 5;22(1):80. doi: 10.1186/s12959-024-00649-w. Thromb J. 2024. PMID: 39237986 Free PMC article.
References
-
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
