Ulcerative Colitis and Acute Severe Ulcerative Colitis Patients Are Overlooked in Infliximab Population Pharmacokinetic Models: Results from a Comprehensive Review
- PMID: 36297530
- PMCID: PMC9610912
- DOI: 10.3390/pharmaceutics14102095
Ulcerative Colitis and Acute Severe Ulcerative Colitis Patients Are Overlooked in Infliximab Population Pharmacokinetic Models: Results from a Comprehensive Review
Abstract
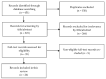
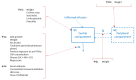
Ulcerative colitis (UC) is part of the inflammatory bowels diseases, and moderate to severe UC patients can be treated with anti-tumour necrosis α monoclonal antibodies, including infliximab (IFX). Even though treatment of UC patients by IFX has been in place for over a decade, many gaps in modelling of IFX PK in this population remain. This is even more true for acute severe UC (ASUC) patients for which early prediction of IFX pharmacokinetic (PK) could highly improve treatment outcome. Thus, this review aims to compile and analyse published population PK models of IFX in UC and ASUC patients, and to assess the current knowledge on disease activity impact on IFX PK. For this, a semi-systematic literature search was conducted, from which 26 publications including a population PK model analysis of UC patients receiving IFX therapy were selected. Amongst those, only four developed a model specifically for UC patients, and only three populations included severe UC patients. Investigations of disease activity impact on PK were reported in only 4 of the 14 models selected. In addition, the lack of reported model codes and assessment of predictive performance make the use of published models in a clinical setting challenging. Thus, more comprehensive investigation of PK in UC and ASUC is needed as well as more adequate reports on developed models and their evaluation in order to apply them in a clinical setting.
Keywords: acute severe ulcerative colitis; disease activity; inflammatory bowel disease; infliximab; pharmacokinetic; pharmacometrics; ulcerative colitis.
Conflict of interest statement
C.K. and W.H. report grants from an industry consortium (AbbVie Deutschland GmbH & Co. KG, AstraZeneca, Boehringer Ingelheim Pharma GmbH & Co. KG, Grünenthal GmbH, F. Hoffmann-La Roche Ltd., Merck KGaA and SANOFI) for the PharMetrX PhD program. C.K. reports grants for the Innovative Medicines Initiative-Joint Undertaking (“DDMoRe”), Diurnal Ltd., the Federal Ministry of Education and Research within the Joint Programming Initiative on Antimicrobial Resistance Initiative (JPIAMR) and from the European Commission within in the Horizon 2020 framework programme (“FAIR”), all outside the submitted work. All other authors declared no competing interests for this work.
Figures

Similar articles
-
Multicenter Cohort Study of Infliximab Pharmacokinetics and Therapy Response in Pediatric Acute Severe Ulcerative Colitis.Clin Gastroenterol Hepatol. 2023 May;21(5):1338-1347. doi: 10.1016/j.cgh.2022.08.016. Epub 2022 Aug 27. Clin Gastroenterol Hepatol. 2023. PMID: 36031093 Free PMC article.
-
Accelerated Clearance of Infliximab is Associated With Treatment Failure in Patients With Corticosteroid-Refractory Acute Ulcerative Colitis.J Crohns Colitis. 2018 May 25;12(6):662-669. doi: 10.1093/ecco-jcc/jjy028. J Crohns Colitis. 2018. PMID: 29659758
-
Post-induction infliximab trough levels and disease activity in the clinical evolution of pediatric ulcerative colitis.United European Gastroenterol J. 2020 May;8(4):425-435. doi: 10.1177/2050640620912877. Epub 2020 Mar 12. United European Gastroenterol J. 2020. PMID: 32213038 Free PMC article.
-
Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262): clinical effectiveness systematic review and economic model.Health Technol Assess. 2016 May;20(39):1-326. doi: 10.3310/hta20390. Health Technol Assess. 2016. PMID: 27220829 Free PMC article. Review.
-
Systematic review with meta-analysis: infliximab and immunosuppressant therapy vs. infliximab alone for active ulcerative colitis.Aliment Pharmacol Ther. 2015 Apr;41(7):603-12. doi: 10.1111/apt.13102. Epub 2015 Feb 10. Aliment Pharmacol Ther. 2015. PMID: 25678223 Review.
Cited by
-
Efficiency of Orexin-A for Inflammatory Flare and Mucosal Healing in Experimental Colitis: Comparison with the Anti-TNF Alpha Infliximab.Int J Mol Sci. 2023 May 31;24(11):9554. doi: 10.3390/ijms24119554. Int J Mol Sci. 2023. PMID: 37298505 Free PMC article.
-
Anti-inflammatory activity of peiminine in acetic acid-induced ulcerative colitis model.Inflammopharmacology. 2024 Feb;32(1):657-665. doi: 10.1007/s10787-023-01360-4. Epub 2023 Oct 19. Inflammopharmacology. 2024. PMID: 37855980
References
-
- Vuitton L., Peyrin-Biroulet L., Colombel J.F., Pariente B., Pineton de Chambrun G., Walsh A.J., Panes J., Travis S.P.L., Mary J.Y., Marteau P. Defining Endoscopic Response and Remission in Ulcerative Colitis Clinical Trials: An International Consensus. Aliment. Pharmacol. Ther. 2017;45:801–813. doi: 10.1111/apt.13948. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

