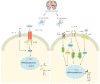
Mechanism and Regulation of Microglia Polarization in Intracerebral Hemorrhage
- PMID: 36296682
- PMCID: PMC9611828
- DOI: 10.3390/molecules27207080
Mechanism and Regulation of Microglia Polarization in Intracerebral Hemorrhage
Abstract
Intracerebral hemorrhage (ICH) is the most lethal subtype of stroke, but effective treatments are lacking, and neuroinflammation plays a key role in the pathogenesis. In the innate immune response to cerebral hemorrhage, microglia first appear around the injured tissue and are involved in the inflammatory cascade response. Microglia respond to acute brain injury by being activated and polarized to either a typical M1-like (pro-inflammatory) or an alternative M2-like (anti-inflammatory) phenotype. These two polarization states produce pro-inflammatory or anti-inflammatory. With the discovery of the molecular mechanisms and key signaling molecules related to the polarization of microglia in the brain, some targets that regulate the polarization of microglia to reduce the inflammatory response are considered a treatment for secondary brain tissue after ICH damage effective strategies. Therefore, how to promote the polarization of microglia to the M2 phenotype after ICH has become the focus of attention in recent years. This article reviews the mechanism of action of microglia's M1 and M2 phenotypes in secondary brain injury after ICH. Moreover, it discusses compounds and natural pharmaceutical ingredients that can polarize the M1 to the M2 phenotype.
Keywords: inflammatory response; intracerebral hemorrhage; microglia polarization; therapeutic target.
Conflict of interest statement
All authors declare that there are no conflict of interest.
Figures
Similar articles
-
Kv1.3 Blockade Alleviates White Matter Injury through Reshaping M1/M2 Phenotypes via the NF-κB Signaling Pathway after Intracerebral Hemorrhage.J Integr Neurosci. 2023 Dec 5;22(6):171. doi: 10.31083/j.jin2206171. J Integr Neurosci. 2023. PMID: 38176920
-
Sinomenine enhances microglia M2 polarization and attenuates inflammatory injury in intracerebral hemorrhage.J Neuroimmunol. 2016 Oct 15;299:28-34. doi: 10.1016/j.jneuroim.2016.08.010. Epub 2016 Aug 9. J Neuroimmunol. 2016. PMID: 27725118
-
MitoQ attenuates brain damage by polarizing microglia towards the M2 phenotype through inhibition of the NLRP3 inflammasome after ICH.Pharmacol Res. 2020 Nov;161:105122. doi: 10.1016/j.phrs.2020.105122. Epub 2020 Aug 10. Pharmacol Res. 2020. PMID: 32791262
-
Neuroinflammation Mediated by NLRP3 Inflammasome After Intracerebral Hemorrhage and Potential Therapeutic Targets.Mol Neurobiol. 2020 Dec;57(12):5130-5149. doi: 10.1007/s12035-020-02082-2. Epub 2020 Aug 27. Mol Neurobiol. 2020. PMID: 32856203 Review.
-
The natural (poly)phenols as modulators of microglia polarization via TLR4/NF-κB pathway exert anti-inflammatory activity in ischemic stroke.Eur J Pharmacol. 2022 Jan 5;914:174660. doi: 10.1016/j.ejphar.2021.174660. Epub 2021 Dec 1. Eur J Pharmacol. 2022. PMID: 34863710 Review.
Cited by
-
New Insights into Roles of IL-7R Gene as a Therapeutic Target Following Intracerebral Hemorrhage.J Inflamm Res. 2024 Jan 18;17:399-415. doi: 10.2147/JIR.S438205. eCollection 2024. J Inflamm Res. 2024. PMID: 38260810 Free PMC article.
-
Ursolic Acid Alleviates Neuroinflammation after Intracerebral Hemorrhage by Mediating Microglial Pyroptosis via the NF-κB/NLRP3/GSDMD Pathway.Int J Mol Sci. 2023 Sep 30;24(19):14771. doi: 10.3390/ijms241914771. Int J Mol Sci. 2023. PMID: 37834220 Free PMC article.
-
Glycometabolic Reprogramming of Microglia in Neurodegenerative Diseases: Insights from Neuroinflammation.Aging Dis. 2024 May 7;15(3):1155-1175. doi: 10.14336/AD.2023.0807. Aging Dis. 2024. PMID: 37611905 Free PMC article. Review.
-
Single-cell RNA sequencing reveals the evolution of the immune landscape during perihematomal edema progression after intracerebral hemorrhage.J Neuroinflammation. 2024 May 28;21(1):140. doi: 10.1186/s12974-024-03113-8. J Neuroinflammation. 2024. PMID: 38807233 Free PMC article.
-
Immune-mediated disruption of the blood-brain barrier after intracerebral hemorrhage: Insights and potential therapeutic targets.CNS Neurosci Ther. 2024 Jul;30(7):e14853. doi: 10.1111/cns.14853. CNS Neurosci Ther. 2024. PMID: 39034473 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- Nos. 81874419, 81673719 and81303074/National Natural ScienceFoundation of China
- No.2017ZX09301064002/National Health Commission of the people's Republic of China
- Nos. ZR2020MB108, ZR2019MH063/Natural Science Foundation of Shandong Province
- NO. 2202112/Youth Innovation Team of Shandong University of Traditional Chinese Medicine
LinkOut - more resources
Full Text Sources
Medical