Inhibitory Effects of Myriocin on Non-Enzymatic Glycation of Bovine Serum Albumin
- PMID: 36296589
- PMCID: PMC9607541
- DOI: 10.3390/molecules27206995
Inhibitory Effects of Myriocin on Non-Enzymatic Glycation of Bovine Serum Albumin
Abstract
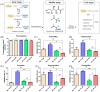
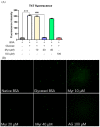
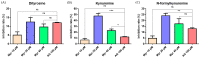
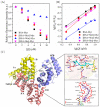
Advanced glycation end products (AGEs) are the compounds produced by non-enzymatic glycation of proteins, which are involved in diabetic-related complications. To investigate the potential anti-glycation activity of Myriocin (Myr), a fungal metabolite of Cordyceps, the effect of Myr on the formation of AGEs resulted from the glycation of bovine serum albumin (BSA) and the interaction between Myr and BSA were studied by multiple spectroscopic techniques and computational simulations. We found that Myr inhibited the formation of AGEs at the end stage of glycation reaction and exhibited strong anti-fibrillation activity. Spectroscopic analysis revealed that Myr quenched the fluorescence of BSA in a static process, with the possible formation of a complex (approximate molar ratio of 1:1). The binding between BSA and Myr mainly depended on van der Waals interaction, hydrophobic interactions and hydrogen bond. The synchronous fluorescence and UV-visible (UV-vis) spectra results indicated that the conformation of BSA altered in the presence of Myr. The fluorescent probe displacement experiments and molecular docking suggested that Myr primarily bound to binding site 1 (subdomain IIA) of BSA. These findings demonstrate that Myr is a potential anti-glycation agent and provide a theoretical basis for the further functional research of Myr in the prevention and treatment of AGEs-related diseases.
Keywords: BSA; Myriocin; computational simulations; non-enzymatic glycation; spectroscopic techniques.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Investigation on the interaction between triclosan and bovine serum albumin by spectroscopic methods.J Environ Sci Health B. 2020;55(1):52-59. doi: 10.1080/03601234.2019.1656499. Epub 2019 Aug 27. J Environ Sci Health B. 2020. PMID: 31453744
-
[Binding interaction of harpagoside and bovine serum albumin: spectroscopic methodologies and molecular docking].Zhongguo Zhong Yao Za Zhi. 2018 Mar;43(5):993-1000. doi: 10.19540/j.cnki.cjcmm.2018.0031. Zhongguo Zhong Yao Za Zhi. 2018. PMID: 29676099 Chinese.
-
Multiple spectroscopic and computational studies on binding interaction of 2-phenylamino-4-phenoxyquinoline derivatives with bovine serum albumin.Spectrochim Acta A Mol Biomol Spectrosc. 2024 Apr 5;310:123948. doi: 10.1016/j.saa.2024.123948. Epub 2024 Jan 23. Spectrochim Acta A Mol Biomol Spectrosc. 2024. PMID: 38309006
-
Inhibitory mechanism of sinensetin on α-glucosidase and non-enzymatic glycation: Insights from spectroscopy and molecular docking analyses.Int J Biol Macromol. 2021 Jan 1;166:259-267. doi: 10.1016/j.ijbiomac.2020.10.174. Epub 2020 Oct 26. Int J Biol Macromol. 2021. PMID: 33115652
-
Insights into the interaction between the kusaginin and bovine serum albumin: Multi-spectroscopic techniques and computational approaches.J Mol Recognit. 2023 Mar;36(3):e3003. doi: 10.1002/jmr.3003. Epub 2022 Dec 23. J Mol Recognit. 2023. PMID: 36519271
Cited by
-
Olea europaea L. Leaves as a Source of Anti-Glycation Compounds.Molecules. 2024 Sep 14;29(18):4368. doi: 10.3390/molecules29184368. Molecules. 2024. PMID: 39339362 Free PMC article.
-
A Role for Advanced Glycation End Products in Molecular Ageing.Int J Mol Sci. 2023 Jun 8;24(12):9881. doi: 10.3390/ijms24129881. Int J Mol Sci. 2023. PMID: 37373042 Free PMC article. Review.
References
-
- Shen C.-Y., Lu C.-H., Wu C.-H., Li K.-J., Kuo Y.-M., Hsieh S.-C., Yu C.-L. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules. 2020;25:5591. doi: 10.3390/molecules25235591. - DOI - PMC - PubMed
-
- Peppa M., Uribarri J., Vlassara H. Glucose, Advanced Glycation End Products, and Diabetes Complications: What Is New and What Works. Clin. Diabetes. 2003;21:186–187. doi: 10.2337/diaclin.21.4.186. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

