Should Reward Deficiency Syndrome (RDS) Be Considered an Umbrella Disorder for Mental Illness and Associated Genetic and Epigenetic Induced Dysregulation of Brain Reward Circuitry?
- PMID: 36294858
- PMCID: PMC9604605
- DOI: 10.3390/jpm12101719
Should Reward Deficiency Syndrome (RDS) Be Considered an Umbrella Disorder for Mental Illness and Associated Genetic and Epigenetic Induced Dysregulation of Brain Reward Circuitry?
Abstract
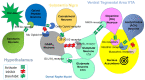
Reward Deficiency Syndrome (RDS) is defined as a breakdown of reward neurotransmission that results in a wide range of addictive, compulsive, and impulsive behaviors. RDS is caused by a combination of environmental (epigenetic) influences and DNA-based (genetic) neurotransmission deficits that interfere with the normal satisfaction of human physiological drives (i.e., food, water, and sex). An essential feature of RDS is the lack of integration between perception, cognition, and emotions that occurs because of (1) significant dopaminergic surges in motivation, reward, and learning centers causing neuroplasticity in the striato-thalamic-frontal cortical loop; (2) hypo-functionality of the excitatory glutamatergic afferents from the amygdala-hippocampus complex. A large volume of literature regarding the known neurogenetic and psychological underpinnings of RDS has revealed a significant risk of dopaminergic gene polymorphic allele overlap between cohorts of depression and subsets of schizophrenia. The suggestion is that instead of alcohol, opioids, gambling disorders, etc. being endophenotypes, the true phenotype is RDS. Additionally, reward deficiency can result from depleted or hereditary hypodopaminergia, which can manifest as a variety of personality traits and mental/medical disorders that have been linked to genetic studies with dopamine-depleting alleles. The carrying of known DNA antecedents, including epigenetic insults, results in a life-long vulnerability to RDS conditions and addictive behaviors. Epigenetic repair of hypodopaminergia, the causative basis of addictive behaviors, may involve precision DNA-guided therapy achieved by combining the Genetic Addiction Risk Severity (GARS) test with a researched neutraceutical having a number of variant names, including KB220Z. This nutraceutical formulation with pro-dopamine regulatory capabilities has been studied and published in peer-reviewed journals, mostly from our laboratory. Finally, it is our opinion that RDS should be given an ICD code and deserves to be included in the DSM-VI because while the DSM features symptomology, it is equally important to feature etiological roots as portrayed in the RDS model.
Keywords: Genetic Addiction Risk Severity (GARS) test; Reward Deficiency Syndrome (RDS); addiction; brain reward cascade; dopamine homeostasis; epigenetics; hypodopaminergia; pro-dopamine regulation (KB220Z).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Reward Deficiency Syndrome (RDS) Surprisingly Is Evolutionary and Found Everywhere: Is It "Blowin' in the Wind"?J Pers Med. 2022 Feb 21;12(2):321. doi: 10.3390/jpm12020321. J Pers Med. 2022. PMID: 35207809 Free PMC article.
-
Coupling Neurogenetics (GARS™) and a Nutrigenomic Based Dopaminergic Agonist to Treat Reward Deficiency Syndrome (RDS): Targeting Polymorphic Reward Genes for Carbohydrate Addiction Algorithms.J Reward Defic Syndr. 2015;1(2):75-80. doi: 10.17756/jrds.2015-012. Epub 2015 Jun 24. J Reward Defic Syndr. 2015. PMID: 27617300 Free PMC article.
-
In Search of Reward Deficiency Syndrome (RDS)-free Controls: The "Holy Grail" in Genetic Addiction Risk Testing.Curr Psychopharmacol. 2020;9(1):7-21. Curr Psychopharmacol. 2020. PMID: 32432025 Free PMC article.
-
A Novel Precision Approach to Overcome the "Addiction Pandemic" by Incorporating Genetic Addiction Risk Severity (GARS) and Dopamine Homeostasis Restoration.J Pers Med. 2021 Mar 16;11(3):212. doi: 10.3390/jpm11030212. J Pers Med. 2021. PMID: 33809702 Free PMC article. Review.
-
Molecular neurological correlates of endorphinergic/dopaminergic mechanisms in reward circuitry linked to endorphinergic deficiency syndrome (EDS).J Neurol Sci. 2020 Apr 15;411:116733. doi: 10.1016/j.jns.2020.116733. Epub 2020 Feb 14. J Neurol Sci. 2020. PMID: 32088516 Review.
Cited by
-
Exercise Modifies the Brain Metabolic Response to Chronic Cocaine Exposure Inhibiting the Stria Terminalis.Brain Sci. 2023 Dec 11;13(12):1705. doi: 10.3390/brainsci13121705. Brain Sci. 2023. PMID: 38137153 Free PMC article.
-
Positive Clinical Outcomes for Severe Reported Pain Using Robust Non-Addictive Home Electrotherapy-A Case-Series.J Pers Med. 2023 Feb 15;13(2):336. doi: 10.3390/jpm13020336. J Pers Med. 2023. PMID: 36836570 Free PMC article.
-
A Complex Relationship Among the Circadian Rhythm, Reward Circuit and Substance Use Disorder (SUD).Psychol Res Behav Manag. 2024 Oct 9;17:3485-3501. doi: 10.2147/PRBM.S473310. eCollection 2024. Psychol Res Behav Manag. 2024. PMID: 39411118 Free PMC article. Review.
-
Genetic Correlates as a Predictor of Bariatric Surgery Outcomes after 1 Year.Biomedicines. 2023 Sep 27;11(10):2644. doi: 10.3390/biomedicines11102644. Biomedicines. 2023. PMID: 37893019 Free PMC article.
-
Diverse Underlying Mechanisms and Sex Differences Found in Translational Models of Cannabinoids Use: Towards Validation in Human Studies.Int J Mol Sci. 2023 Nov 22;24(23):16586. doi: 10.3390/ijms242316586. Int J Mol Sci. 2023. PMID: 38068909 Free PMC article.
References
-
- Blum K., Bowirrat A., Braverman E.R., Baron D., Cadet J.L., Kazmi S., Elman I., Thanos P.K., Badgaiyan R.D., Downs W.B., et al. Reward Deficiency Syndrome (RDS): A Cytoarchitectural Common Neurobiological Trait of All Addictions. Int. J. Environ. Res. Public Health. 2021;18:11529. doi: 10.3390/ijerph182111529. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

