Microbe-Derived Antioxidants Reduce Lipopolysaccharide-Induced Inflammatory Responses by Activating the Nrf2 Pathway to Inhibit the ROS/NLRP3/IL-1β Signaling Pathway
- PMID: 36293333
- PMCID: PMC9603940
- DOI: 10.3390/ijms232012477
Microbe-Derived Antioxidants Reduce Lipopolysaccharide-Induced Inflammatory Responses by Activating the Nrf2 Pathway to Inhibit the ROS/NLRP3/IL-1β Signaling Pathway
Abstract
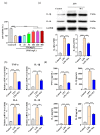
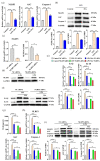
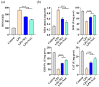
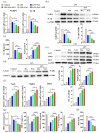
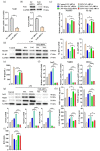
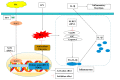
Inflammation plays an important role in the innate immune response, yet overproduction of inflammation can lead to a variety of chronic diseases associated with the innate immune system; therefore, modulation of the excessive inflammatory response has been considered a major strategy in the treatment of inflammatory diseases. Activation of the ROS/NLRP3/IL-1β signaling axis has been suggested to be a key initiating phase of inflammation. Our previous study found that microbe-derived antioxidants (MA) are shown to have excellent antioxidant and anti-inflammatory properties; however, the mechanism of action of MA remains unclear. The current study aims to investigate whether MA could protect cells from LPS-induced oxidative stress and inflammatory responses by modulating the Nrf2-ROS-NLRP3-IL-1β signaling pathway. In this study, we find that MA treatment significantly alleviates LPS-induced oxidative stress and inflammatory responses in RAW264.7 cells. MA significantly reduce the accumulation of ROS in RAW264.7 cells, down-regulate the levels of pro-inflammatory factors (TNF-α and IL-6), inhibit NLRP3, ASC, caspase-1 mRNA, and protein levels, and reduce the mRNA, protein levels, and content of inflammatory factors (IL-1β and IL-18). The protective effect of MA is significantly reduced after the siRNA knockdown of the NLRP3 gene, presumably related to the ability of MA to inhibit the ROS-NLRP3-IL-1β signaling pathway. MA is able to reduce the accumulation of ROS and alleviate oxidative stress by increasing the content of antioxidant enzymes, such as SOD, GSH-Px, and CAT. The protective effect of MA may be due to its ability of MA to induce Nrf2 to enter the nucleus and initiate the expression of antioxidant enzymes. The antioxidant properties of MA are further enhanced in the presence of the Nrf2 activator SFN. After the siRNA knockdown of the Nrf2 gene, the antioxidant and anti-inflammatory properties of MA are significantly affected. These findings suggest that MA may inhibit the LPS-stimulated ROS/NLRP3/IL-1β signaling axis by activating Nrf2-antioxidant signaling in RAW264.7 cells. As a result of this study, MA has been found to alleviate inflammatory responses and holds promise as a therapeutic agent for inflammation-related diseases.
Keywords: Nrf2; ROS/NLRP3/IL-1β; inflammatory response; microbial-derived antioxidants; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Microbe-Derived Antioxidants Protect IPEC-1 Cells from H2O2-Induced Oxidative Stress, Inflammation and Tight Junction Protein Disruption via Activating the Nrf2 Pathway to Inhibit the ROS/NLRP3/IL-1β Signaling Pathway.Antioxidants (Basel). 2024 Apr 27;13(5):533. doi: 10.3390/antiox13050533. Antioxidants (Basel). 2024. PMID: 38790638 Free PMC article.
-
Syringic acid suppresses Cutibacterium acnes-induced inflammation in human keratinocytes via regulating the NLRP3/caspase-1/IL-1β signaling axis by activating PPARγ/Nrf2-antioxidant pathway.Int Immunopharmacol. 2024 Sep 30;139:112708. doi: 10.1016/j.intimp.2024.112708. Epub 2024 Jul 20. Int Immunopharmacol. 2024. PMID: 39033661
-
Glycyrrhizin alleviates varicellovirus bovinealpha 1-induced oxidative stress, inflammation, and apoptosis in MDBK cells by inhibiting NF-κB/NLRP3 axis through the Nrf2 signalling pathway.Vet Res Commun. 2024 Apr;48(2):749-759. doi: 10.1007/s11259-023-10242-7. Epub 2023 Oct 27. Vet Res Commun. 2024. PMID: 37889426
-
Crosstalk between Nrf2 signaling pathway and inflammation in ischemic stroke: Mechanisms of action and therapeutic implications.Exp Neurol. 2024 Mar;373:114655. doi: 10.1016/j.expneurol.2023.114655. Epub 2023 Dec 17. Exp Neurol. 2024. PMID: 38110142 Review.
-
Targeting NLRP3 Inflammasome With Nrf2 Inducers in Central Nervous System Disorders.Front Immunol. 2022 Mar 28;13:865772. doi: 10.3389/fimmu.2022.865772. eCollection 2022. Front Immunol. 2022. PMID: 35418995 Free PMC article. Review.
Cited by
-
Effects of the compound extracts of Caprifoliaceae and Scutellaria baicalensis Georgi on the intestinal microbiota and antioxidant function.Front Microbiol. 2024 Jan 12;14:1289490. doi: 10.3389/fmicb.2023.1289490. eCollection 2023. Front Microbiol. 2024. PMID: 38282732 Free PMC article.
-
Research progress of sea buckthorn (Hippophae rhamnoides L.) in prevention and treatment of cardiovascular disease.Front Cardiovasc Med. 2024 Oct 18;11:1477636. doi: 10.3389/fcvm.2024.1477636. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39494241 Free PMC article. Review.
-
The Gut-Brain Axis as a Therapeutic Target in Multiple Sclerosis.Cells. 2023 Jul 17;12(14):1872. doi: 10.3390/cells12141872. Cells. 2023. PMID: 37508537 Free PMC article. Review.
-
Microbe-Derived Antioxidants Alleviate Liver and Adipose Tissue Lipid Disorders and Metabolic Inflammation Induced by High Fat Diet in Mice.Int J Mol Sci. 2023 Feb 7;24(4):3269. doi: 10.3390/ijms24043269. Int J Mol Sci. 2023. PMID: 36834674 Free PMC article.
-
Antioxidative Sirt1 and the Keap1-Nrf2 Signaling Pathway Impair Inflammation and Positively Regulate Autophagy in Murine Mammary Epithelial Cells or Mammary Glands Infected with Streptococcus uberis.Antioxidants (Basel). 2024 Jan 29;13(2):171. doi: 10.3390/antiox13020171. Antioxidants (Basel). 2024. PMID: 38397769 Free PMC article.
References
-
- Hseu Y.C., Tseng Y.F., Pandey S., Shrestha S., Lin K.Y., Lin C.W., Lee C.C., Huang S.T., Yang H.L. Coenzyme Q0 Inhibits NLRP3 Inflammasome Activation through Mitophagy Induction in LPS/ATP-Stimulated Macrophages. Oxid. Med. Cell. Longev. 2022;2022:4266214. doi: 10.1155/2022/4266214. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

