Epigenetic Regulation by microRNAs in Hyperhomocysteinemia-Accelerated Atherosclerosis
- PMID: 36293305
- PMCID: PMC9604464
- DOI: 10.3390/ijms232012452
Epigenetic Regulation by microRNAs in Hyperhomocysteinemia-Accelerated Atherosclerosis
Abstract
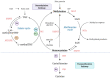
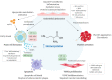
Increased serum levels of homocysteine (Hcy) is a risk factor for cardiovascular disease and is specifically linked to various diseases of the vasculature such as atherosclerosis. However, the precise mechanisms by which Hcy contributes to this condition remain elusive. During the development of atherosclerosis, epigenetic modifications influence gene expression. As such, epigenetic modifications are an adaptive response to endogenous and exogenous factors that lead to altered gene expression by methylation and acetylation reactions of different substrates and the action of noncoding RNA including microRNAs (miRNAs). Epigenetic remodeling modulates cell biology in both physiological and physiopathological conditions. DNA and histone modification have been identified to have a crucial role in the progression of atherosclerosis. However, the potential role of miRNAs in hyperHcy (HHcy)-related atherosclerosis disease remains poorly explored and might be essential as well. There is no review available yet summarizing the contribution of miRNAs to hyperhomocystein-mediated atherogenicity or their potential as therapeutic targets even though their important role has been described in numerous studies. Specifically, downregulation of miR-143 or miR-125b has been shown to regulate VSCMs proliferation in vitro. In preclinical studies, downregulation of miR-92 or miR195-3p has been shown to increase the accumulation of cholesterol in foam cells and increase macrophage inflammation and atherosclerotic plaque formation, respectively. Another preclinical study found that there is a reciprocal regulation between miR-148a/152 and DNMT1 in Hcy-accelerated atherosclerosis. Interestingly, a couple of studies have shown that miR-143 or miR-217 may be used as potential biomarkers in patients with HHcy that may develop atherosclerosis. Moreover, the current review will also update current knowledge on miRNA-based therapies, their challenges, and approaches to deal with Hcy-induced atherosclerosis.
Keywords: endothelial cells; hyperhomocysteinemia; macrophages; microRNAs; vascular smooth muscle cells and atherosclerosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Reciprocal Regulation Between miR-148a/152 and DNA Methyltransferase 1 Is Associated with Hyperhomocysteinemia-Accelerated Atherosclerosis.DNA Cell Biol. 2017 Jun;36(6):462-474. doi: 10.1089/dna.2017.3651. Epub 2017 May 4. DNA Cell Biol. 2017. PMID: 28472596
-
miR-34a Targets HDAC1-Regulated H3K9 Acetylation on Lipid Accumulation Induced by Homocysteine in Foam Cells.J Cell Biochem. 2017 Dec;118(12):4617-4627. doi: 10.1002/jcb.26126. Epub 2017 Jun 12. J Cell Biochem. 2017. PMID: 28485501
-
Expression levels of atherosclerosis-associated miR-143 and miR-145 in the plasma of patients with hyperhomocysteinaemia.BMC Cardiovasc Disord. 2017 Jun 20;17(1):163. doi: 10.1186/s12872-017-0596-0. BMC Cardiovasc Disord. 2017. PMID: 28633641 Free PMC article.
-
Synergy of homocysteine, microRNA, and epigenetics: a novel therapeutic approach for stroke.Mol Neurobiol. 2013 Aug;48(1):157-68. doi: 10.1007/s12035-013-8421-y. Epub 2013 Feb 22. Mol Neurobiol. 2013. PMID: 23430482 Free PMC article. Review.
-
Dysregulation of Epigenetic Mechanisms of Gene Expression in the Pathologies of Hyperhomocysteinemia.Int J Mol Sci. 2019 Jun 27;20(13):3140. doi: 10.3390/ijms20133140. Int J Mol Sci. 2019. PMID: 31252610 Free PMC article. Review.
Cited by
-
The role of the mitochondrial trans-sulfuration in cerebro-cardio renal dysfunction during trisomy down syndrome.Mol Cell Biochem. 2024 Apr;479(4):825-829. doi: 10.1007/s11010-023-04761-9. Epub 2023 May 17. Mol Cell Biochem. 2024. PMID: 37198322 Review.
-
MicroRNAs Regulate Function in Atherosclerosis and Clinical Implications.Oxid Med Cell Longev. 2023 Aug 29;2023:2561509. doi: 10.1155/2023/2561509. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 37675243 Free PMC article. Review.
-
Valsartan Attenuated Homocysteine-Induced Impaired Autophagy and ER Stress in Human Umbilical Vein Endothelial Cells.Cardiovasc Ther. 2023 Dec 13;2023:8817431. doi: 10.1155/2023/8817431. eCollection 2023. Cardiovasc Ther. 2023. PMID: 38125704 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

