Targeting Deubiquitinating Enzymes (DUBs) That Regulate Mitophagy via Direct or Indirect Interaction with Parkin
- PMID: 36292958
- PMCID: PMC9603086
- DOI: 10.3390/ijms232012105
Targeting Deubiquitinating Enzymes (DUBs) That Regulate Mitophagy via Direct or Indirect Interaction with Parkin
Abstract
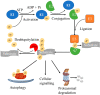
The quality control of mitochondria is critical for the survival of cells, and defects in the pathways required for this quality control can lead to severe disease. A key quality control mechanism in cells is mitophagy, which functions to remove damaged mitochondria under conditions of various stresses. Defective mitophagy can lead to a number of diseases including neurodegeneration. It has been proposed that an enhancement of mitophagy can improve cell survival, enhance neuronal function in neurodegeneration and extend health and lifespans. In this review, we highlight the role of deubiquitinating enzymes (DUBs) in the regulation of mitophagy. We summarise the current knowledge on DUBs that regulate mitophagy as drug targets and provide a list of small molecule inhibitors that are valuable tools for the further development of therapeutic strategies targeting the mitophagy pathway in neurodegeneration.
Keywords: DUB inhibitors; Parkin; Parkinson disease; deubiquitinating enzymes; mitophagy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
USP33 deubiquitinates PRKN/parkin and antagonizes its role in mitophagy.Autophagy. 2020 Apr;16(4):724-734. doi: 10.1080/15548627.2019.1656957. Epub 2019 Aug 26. Autophagy. 2020. PMID: 31432739 Free PMC article.
-
Role of Deubiquitinases in Parkinson's Disease-Therapeutic Perspectives.Cells. 2023 Feb 17;12(4):651. doi: 10.3390/cells12040651. Cells. 2023. PMID: 36831318 Free PMC article. Review.
-
The three 'P's of mitophagy: PARKIN, PINK1, and post-translational modifications.Genes Dev. 2015 May 15;29(10):989-99. doi: 10.1101/gad.262758.115. Genes Dev. 2015. PMID: 25995186 Free PMC article. Review.
-
USP8 Down-Regulation Promotes Parkin-Independent Mitophagy in the Drosophila Brain and in Human Neurons.Cells. 2023 Apr 13;12(8):1143. doi: 10.3390/cells12081143. Cells. 2023. PMID: 37190052 Free PMC article.
-
USP8 regulates mitophagy by removing K6-linked ubiquitin conjugates from parkin.EMBO J. 2014 Nov 3;33(21):2473-91. doi: 10.15252/embj.201489729. Epub 2014 Sep 12. EMBO J. 2014. PMID: 25216678 Free PMC article.
Cited by
-
USP26 promotes colorectal cancer tumorigenesis by restraining PRKN-mediated mitophagy.Oncogene. 2024 May;43(21):1581-1593. doi: 10.1038/s41388-024-03009-0. Epub 2024 Apr 2. Oncogene. 2024. PMID: 38565942
-
VDR and deubiquitination control neuronal oxidative stress and microglial inflammation in Parkinson's disease.Cell Death Discov. 2024 Mar 21;10(1):150. doi: 10.1038/s41420-024-01912-9. Cell Death Discov. 2024. PMID: 38514643 Free PMC article.
-
PRKN-linked familial Parkinson's disease: cellular and molecular mechanisms of disease-linked variants.Cell Mol Life Sci. 2024 May 20;81(1):223. doi: 10.1007/s00018-024-05262-8. Cell Mol Life Sci. 2024. PMID: 38767677 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

