Immunomodulation by Hemoadsorption-Changes in Hepatic Biotransformation Capacity in Sepsis and Septic Shock: A Prospective Study
- PMID: 36289602
- PMCID: PMC9598581
- DOI: 10.3390/biomedicines10102340
Immunomodulation by Hemoadsorption-Changes in Hepatic Biotransformation Capacity in Sepsis and Septic Shock: A Prospective Study
Abstract
Background: Sepsis is often associated with liver dysfunction, which is an indicator of poor outcomes. Specific diagnostic tools that detect hepatic dysfunction in its early stages are scarce. So far, the immune modulatory effects of hemoadsorption with CytoSorb® on liver function are unclear.
Method: We assessed the hepatic function by using the dynamic LiMAx® test and biochemical parameters in 21 patients with sepsis or septic shock receiving CytoSorb® in a prospective, observational study. Points of measurement: T1: diagnosis of sepsis or septic shock; T2 and T3: 24 h and 48 h after the start of CytoSorb®; T4: 24 h after termination of CytoSorb®.
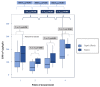
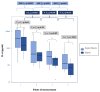
Results: The hepatic biotransformation capacity measured by LiMAx® was severely impaired in up to 95 % of patients. Despite a rapid shock reversal under CytoSorb®, a significant improvement in LiMAx® values appeared from T3 to T4. This decline and recovery of liver function were not reflected by common parameters of hepatic metabolism that remained mostly within the normal range.
Conclusions: Hepatic dysfunction can effectively and safely be diagnosed with LiMAx® in ventilated ICU patients under CytoSorb®. Various static liver parameters are of limited use since they do not adequately reflect hepatic dysfunction and impaired hepatic metabolism.
Keywords: CytoSorb®; Interleukin-6; LiMAx®; dynamic liver function test; hemoadsorption; hepatic dysfunction; sepsis; sepsis-associated liver dysfunction; septic shock; static liver parameters.
Conflict of interest statement
TK had received lecture fees from Cytosorbent Europe. The Department of Anesthesiology at Klinikum Herford received an unrestricted research grant from Cytosorbent Europe in 2018. The authors declare that they have no other competing interests.
Figures
Similar articles
-
Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (CytoSorb®) in patients with sepsis and septic shock.World J Crit Care Med. 2021 Jan 9;10(1):22-34. doi: 10.5492/wjccm.v10.i1.22. eCollection 2021 Jan 9. World J Crit Care Med. 2021. PMID: 33505870 Free PMC article.
-
Early diagnosis of sepsis-related hepatic dysfunction and its prognostic impact on survival: a prospective study with the LiMAx test.Crit Care. 2013 Oct 31;17(5):R259. doi: 10.1186/cc13089. Crit Care. 2013. PMID: 24172237 Free PMC article.
-
First Evaluation of a New Dynamic Scoring System Intended to Support Prescription of Adjuvant CytoSorb Hemoadsorption Therapy in Patients with Septic Shock.J Clin Med. 2021 Jun 30;10(13):2939. doi: 10.3390/jcm10132939. J Clin Med. 2021. PMID: 34209001 Free PMC article.
-
Therapeutic Modulation of the Host Defense by Hemoadsorption with CytoSorb®-Basics, Indications and Perspectives-A Scoping Review.Int J Mol Sci. 2021 Nov 26;22(23):12786. doi: 10.3390/ijms222312786. Int J Mol Sci. 2021. PMID: 34884590 Free PMC article. Review.
-
Blood purification in sepsis and COVID-19: what´s new in cytokine and endotoxin hemoadsorption.J Anesth Analg Crit Care. 2022 Apr 4;2(1):15. doi: 10.1186/s44158-022-00043-w. J Anesth Analg Crit Care. 2022. PMID: 37386575 Free PMC article. Review.
Cited by
-
Isoflurane, like sepsis, decreases CYP1A2 liver enzyme activity in intensive care patients: a clinical study and network model.Intensive Care Med Exp. 2024 Apr 8;12(1):33. doi: 10.1186/s40635-024-00617-8. Intensive Care Med Exp. 2024. PMID: 38589754 Free PMC article.
-
Hemoadsorption Therapy for Critically Ill Patients with Acute Liver Dysfunction: A Meta-Analysis and Systematic Review.Biomedicines. 2023 Dec 27;12(1):67. doi: 10.3390/biomedicines12010067. Biomedicines. 2023. PMID: 38255174 Free PMC article. Review.
References
-
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

