Spinal microglia contribute to sustained inflammatory pain via amplifying neuronal activity
- PMID: 36289499
- PMCID: PMC9609165
- DOI: 10.1186/s13041-022-00970-3
Spinal microglia contribute to sustained inflammatory pain via amplifying neuronal activity
Abstract
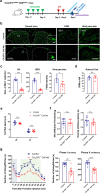
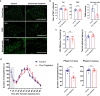
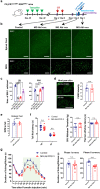
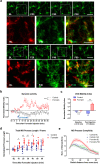
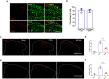
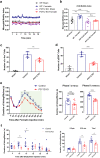
Microglia are highly dynamic immune cells of the central nervous system (CNS). Microglial processes interact with neuronal elements constantly on the order of minutes. The functional significance of this acute microglia-neuron interaction and its potential role in the context of pain is still largely unknown. Here, we found that spinal microglia increased their process motility and electrophysiological reactivity within an hour after the insult in a mouse model of formalin-induced acute, sustained, inflammatory pain. Using an ablation strategy to specifically deplete resident microglia in the CNS, we demonstrate that microglia participate in formalin-induced acute sustained pain behaviors by amplifying neuronal activity in the spinal dorsal horn. Moreover, we identified that the P2Y12 receptor, which is specifically expressed in microglia in the CNS, was required for microglial function in formalin-induced pain. Taken together, our study provides a novel insight into the contribution of microglia and the P2Y12 receptor in inflammatory pain that could be used for potential therapeutic strategies.
Keywords: Formalin; Inflammatory pain; Microglia; Microglia–neuron interaction; P2Y12 receptor; Two-photon imaging.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain.Brain Behav Immun. 2016 Jul;55:82-92. doi: 10.1016/j.bbi.2015.11.007. Epub 2015 Nov 11. Brain Behav Immun. 2016. PMID: 26576724 Free PMC article.
-
Gut-innervating TRPV1+ Neurons Drive Chronic Visceral Pain via Microglial P2Y12 Receptor.Cell Mol Gastroenterol Hepatol. 2022;13(4):977-999. doi: 10.1016/j.jcmgh.2021.12.012. Epub 2021 Dec 24. Cell Mol Gastroenterol Hepatol. 2022. PMID: 34954381 Free PMC article.
-
P2Y12 regulates microglia activation and excitatory synaptic transmission in spinal lamina II neurons during neuropathic pain in rodents.Cell Death Dis. 2019 Feb 18;10(3):165. doi: 10.1038/s41419-019-1425-4. Cell Death Dis. 2019. PMID: 30778044 Free PMC article.
-
Neuron-microglia interaction by purinergic signaling in neuropathic pain following neurodegeneration.Neuropharmacology. 2016 May;104:76-81. doi: 10.1016/j.neuropharm.2015.08.042. Epub 2015 Aug 29. Neuropharmacology. 2016. PMID: 26327676 Review.
-
Purinergic systems, neuropathic pain and the role of microglia.Exp Neurol. 2012 Apr;234(2):293-301. doi: 10.1016/j.expneurol.2011.09.016. Epub 2011 Sep 17. Exp Neurol. 2012. PMID: 21946271 Review.
Cited by
-
Multiphoton fluorescence microscopy for in vivo imaging.Cell. 2024 Aug 22;187(17):4458-4487. doi: 10.1016/j.cell.2024.07.036. Cell. 2024. PMID: 39178829 Review.
-
Microglial inflammation modulates opioid analgesic tolerance.J Neurosci Res. 2023 Sep;101(9):1383-1392. doi: 10.1002/jnr.25199. Epub 2023 Apr 26. J Neurosci Res. 2023. PMID: 37186407 Free PMC article. Review.
-
Biphasic Hormetic-like Effect of Lebecetin, a C-type Lectin of Snake Venom, on Formalin-induced Inflammation in Mice.Curr Neuropharmacol. 2024;22(8):1391-1405. doi: 10.2174/1570159X22999231207105743. Curr Neuropharmacol. 2024. PMID: 38073106 Free PMC article.
-
Mirtazapine Improves Locomotor Activity and Attenuates Neuropathic Pain Following Spinal Cord Injury in Rats via Neuroinflammation Modulation.Neurochem Res. 2024 Dec;49(12):3326-3341. doi: 10.1007/s11064-024-04240-7. Epub 2024 Sep 13. Neurochem Res. 2024. PMID: 39271550
-
Dehydrocorydaline alleviates sleep deprivation-induced persistent postoperative pain in adolescent mice through inhibiting microglial P2Y12 receptor expression in the spinal cord.Mol Pain. 2023 Jan-Dec;19:17448069231216234. doi: 10.1177/17448069231216234. Mol Pain. 2023. PMID: 37940138 Free PMC article.
References
-
- Wolf SA, Boddeke HW, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. 2017;79:619–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

