Matrix Gla protein (MGP), GATA3, and TRPS1: a novel diagnostic panel to determine breast origin
- PMID: 36284362
- PMCID: PMC9598034
- DOI: 10.1186/s13058-022-01569-1
Matrix Gla protein (MGP), GATA3, and TRPS1: a novel diagnostic panel to determine breast origin
Abstract
Background: Metastatic breast carcinoma is commonly considered during differential diagnosis when metastatic disease is detected in females. In addition to the tumor morphology and documented clinical history, sensitive and specific immunohistochemical (IHC) markers such as GCDFP-15, mammaglobin, and GATA3 are helpful for determining breast origin. However, these markers are reported to show lower sensitivity in certain subtypes, such as triple-negative breast cancer (TNBC).
Materials and methods: Using bioinformatics analyses, we identified a potential diagnostic panel to determine breast origin: matrix Gla protein (MGP), transcriptional repressor GATA binding 1 (TRPS1), and GATA-binding protein 3 (GATA3). We compared MGP, TRPS1, and GATA3 expression in different subtypes of breast carcinoma of (n = 1201) using IHC. As a newly identified marker, MGP expression was also evaluated in solid tumors (n = 2384) and normal tissues (n = 1351) from different organs.
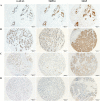
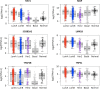
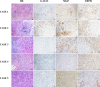
Results: MGP and TRPS1 had comparable positive expression in HER2-positive (91.2% vs. 92.0%, p = 0.79) and TNBC subtypes (87.3% vs. 91.2%, p = 0.18). GATA3 expression was lower than MGP (p < 0.001) or TRPS1 (p < 0.001), especially in HER2-positive (77.0%, p < 0.001) and TNBC (43.3%, p < 0.001) subtypes. TRPS1 had the highest positivity rate (97.9%) in metaplastic TNBCs, followed by MGP (88.6%), while only 47.1% of metaplastic TNBCs were positive for GATA3. When using MGP, GATA3, and TRPS1 as a novel IHC panel, 93.0% of breast carcinomas were positive for at least two markers, and only 9 cases were negative for all three markers. MGP was detected in 36 cases (3.0%) that were negative for both GATA3 and TRPS1. MGP showed mild-to-moderate positive expression in normal hepatocytes, renal tubules, as well as 31.1% (99/318) of hepatocellular carcinomas. Rare cases (0.6-5%) had focal MGP expression in renal, ovarian, lung, urothelial, and cholangiocarcinomas.
Conclusions: Our findings suggest that MGP is a newly identified sensitive IHC marker to support breast origin. MGP, TRPS1, and GATA3 could be applied as a reliable diagnostic panel to determine breast origin in clinical practice.
Keywords: Breast carcinoma; GATA3; Immunohistochemical; MGP; TRPS1.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Diagnostic utility and sensitivities of matrix Gla protein (MGP), TRPS1 and GATA3 in breast cancer: focusing on metastatic breast cancer, invasive breast carcinoma with special features, and salivary gland-type tumours.Pathology. 2024 Jun;56(4):516-527. doi: 10.1016/j.pathol.2024.01.003. Epub 2024 Mar 14. Pathology. 2024. PMID: 38570266
-
Comparative expression of TRPS1, GATA3, SOX10, mammaglobin, and GCDFP-15 in effusion specimens with breast carcinoma.Diagn Cytopathol. 2023 Nov;51(11):665-673. doi: 10.1002/dc.25195. Epub 2023 Jul 17. Diagn Cytopathol. 2023. PMID: 37461248
-
TRPS1, GATA3, and SOX10 expression in triple-negative breast carcinoma.Hum Pathol. 2022 Jul;125:97-107. doi: 10.1016/j.humpath.2022.04.006. Epub 2022 Apr 9. Hum Pathol. 2022. PMID: 35413381
-
The Novel Marker GATA3 is Significantly More Sensitive Than Traditional Markers Mammaglobin and GCDFP15 for Identifying Breast Cancer in Surgical and Cytology Specimens of Metastatic and Matched Primary Tumors.Appl Immunohistochem Mol Morphol. 2016 Apr;24(4):229-37. doi: 10.1097/PAI.0000000000000186. Appl Immunohistochem Mol Morphol. 2016. PMID: 25906123 Free PMC article. Review.
-
Immunohistochemical Markers for Distinguishing Metastatic Breast Carcinoma from Other Common Malignancies: Update and Revisit.Semin Diagn Pathol. 2022 Sep;39(5):313-321. doi: 10.1053/j.semdp.2022.04.002. Epub 2022 Apr 14. Semin Diagn Pathol. 2022. PMID: 35461734 Review.
Cited by
-
TRPS1, a sensitive marker for different histological and molecular types of breast cancer.Diagn Pathol. 2024 Sep 6;19(1):121. doi: 10.1186/s13000-024-01542-w. Diagn Pathol. 2024. PMID: 39243111 Free PMC article.
-
TRPS1 is a Highly Sensitive Marker for Breast Cancer: A Tissue Microarray Study Evaluating More Than 19,000 Tumors From 152 Different Tumor Entities.Am J Surg Pathol. 2024 Jun 1;48(6):637-651. doi: 10.1097/PAS.0000000000002213. Epub 2024 Apr 18. Am J Surg Pathol. 2024. PMID: 38647255 Free PMC article.
-
TRPS1 expression in primary and metastatic prostatic adenocarcinoma, muscle invasive bladder urothelial carcinoma, and breast carcinoma: Is TRPS1 truly specific and sensitive for a breast primary?Hum Pathol. 2024 Jan;143:42-49. doi: 10.1016/j.humpath.2023.11.012. Epub 2023 Dec 3. Hum Pathol. 2024. PMID: 38052269 Free PMC article.
-
A Comprehensive Review of TRPS1 as a Diagnostic Immunohistochemical Marker for Primary Breast Carcinoma: Latest Insights and Diagnostic Pitfalls.Cancers (Basel). 2024 Oct 23;16(21):3568. doi: 10.3390/cancers16213568. Cancers (Basel). 2024. PMID: 39518009 Free PMC article. Review.
-
TRPS1 expression in breast angiosarcoma.Virchows Arch. 2024 Jun 20. doi: 10.1007/s00428-024-03852-2. Online ahead of print. Virchows Arch. 2024. PMID: 38902365
References
-
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2018, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2018/.
-
- Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers. 2019;5(1):66. - PubMed
-
- Gown AM, Fulton RS, Kandalaft PL. Markers of metastatic carcinoma of breast origin. Histopathology. 2016;68(1):86–95. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

