Co-alterations of circadian clock gene transcripts in human placenta in preeclampsia
- PMID: 36284122
- PMCID: PMC9596722
- DOI: 10.1038/s41598-022-22507-3
Co-alterations of circadian clock gene transcripts in human placenta in preeclampsia
Abstract
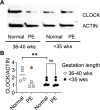
Pre-eclampsia (PE) is a hypertensive condition that occurs during pregnancy and complicates up to 4% of pregnancies. PE exhibits several circadian-related characteristics, and the placenta possesses a functioning molecular clock. We examined the associations of 17 core circadian gene transcripts in placenta with PE vs. non-PE (a mixture of pregnant women with term, preterm, small-for-gestational-age, or chorioamnionitis) using two independent gene expression datasets: GSE75010-157 (80 PE vs. 77 non-PE) and GSE75010-173 (77 PE and 96 non-PE). We found a robust difference in circadian gene expression between PE and non-PE across the two datasets, where CRY1 mRNA increases and NR1D2 and PER3 transcripts decrease in PE placenta. Gene set variation analysis revealed an interplay between co-alterations of circadian clock genes and PE with altered hypoxia, cell migration/invasion, autophagy, and membrane trafficking pathways. Using human placental trophoblast HTR-8 cells, we show that CRY1/2 and NR1D1/2 regulate trophoblast migration. A subgroup study including only term samples demonstrated that CLOCK, NR1D2, and PER3 transcripts were simultaneously decreased in PE placenta, a finding supported by CLOCK protein downregulation in an independent cohort of human term PE placenta samples. These findings provide novel insights into the roles of the molecular clock in the pathogenesis of PE.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Disruption of the Expression of the Placental Clock and Melatonin Genes in Preeclampsia.Int J Mol Sci. 2023 Jan 25;24(3):2363. doi: 10.3390/ijms24032363. Int J Mol Sci. 2023. PMID: 36768691 Free PMC article.
-
Early- and late-onset preeclampsia and the DNA methylation of circadian clock and clock-controlled genes in placental and newborn tissues.Chronobiol Int. 2017;34(7):921-932. doi: 10.1080/07420528.2017.1326125. Epub 2017 Jun 14. Chronobiol Int. 2017. PMID: 28613964
-
Circadian clock gene Clock is involved in the pathogenesis of preeclampsia through hypoxia.Life Sci. 2020 Apr 15;247:117441. doi: 10.1016/j.lfs.2020.117441. Epub 2020 Feb 17. Life Sci. 2020. PMID: 32074481
-
A rhythmic placenta? Circadian variation, clock genes and placental function.Placenta. 2012 Jul;33(7):533-9. doi: 10.1016/j.placenta.2012.03.008. Epub 2012 Apr 22. Placenta. 2012. PMID: 22525887 Review.
-
The Circadian Clock, Nutritional Signals and Reproduction: A Close Relationship.Int J Mol Sci. 2023 Jan 12;24(2):1545. doi: 10.3390/ijms24021545. Int J Mol Sci. 2023. PMID: 36675058 Free PMC article. Review.
Cited by
-
Disruption of the Expression of the Placental Clock and Melatonin Genes in Preeclampsia.Int J Mol Sci. 2023 Jan 25;24(3):2363. doi: 10.3390/ijms24032363. Int J Mol Sci. 2023. PMID: 36768691 Free PMC article.
-
Circadian Disruption and the Molecular Clock in Atherosclerosis and Hypertension.Can J Cardiol. 2023 Dec;39(12):1757-1771. doi: 10.1016/j.cjca.2023.06.416. Epub 2023 Jun 22. Can J Cardiol. 2023. PMID: 37355229 Free PMC article. Review.
-
Placental circadian lincRNAs and spontaneous preterm birth.Front Genet. 2023 Jan 11;13:1051396. doi: 10.3389/fgene.2022.1051396. eCollection 2022. Front Genet. 2023. PMID: 36712876 Free PMC article.
-
Exposure to unmethylated CpG oligonucleotides disrupts blood pressure circadian rhythms and placental clock gene network in pregnant rats.Am J Physiol Heart Circ Physiol. 2023 Aug 1;325(2):H323-H337. doi: 10.1152/ajpheart.00154.2023. Epub 2023 Jun 23. Am J Physiol Heart Circ Physiol. 2023. PMID: 37352412 Free PMC article.
-
MicroRNAs in the Pathogenesis of Preeclampsia-A Case-Control In Silico Analysis.Curr Issues Mol Biol. 2024 Apr 17;46(4):3438-3459. doi: 10.3390/cimb46040216. Curr Issues Mol Biol. 2024. PMID: 38666946 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

