mTORC1 beyond anabolic metabolism: Regulation of cell death
- PMID: 36282248
- PMCID: PMC9606688
- DOI: 10.1083/jcb.202208103
mTORC1 beyond anabolic metabolism: Regulation of cell death
Abstract
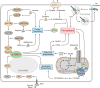
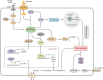
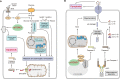
The mechanistic target of rapamycin complex 1 (mTORC1), a multi-subunit protein kinase complex, interrogates growth factor signaling with cellular nutrient and energy status to control metabolic homeostasis. Activation of mTORC1 promotes biosynthesis of macromolecules, including proteins, lipids, and nucleic acids, and simultaneously suppresses catabolic processes such as lysosomal degradation of self-constituents and extracellular components. Metabolic regulation has emerged as a critical determinant of various cellular death programs, including apoptosis, pyroptosis, and ferroptosis. In this article, we review the expanding knowledge on how mTORC1 coordinates metabolic pathways to impinge on cell death regulation. We focus on the current understanding on how nutrient status and cellular signaling pathways connect mTORC1 activity with ferroptosis, an iron-dependent cell death program that has been implicated in a plethora of human diseases. In-depth understanding of the principles governing the interaction between mTORC1 and cell death pathways can ultimately guide the development of novel therapies for the treatment of relevant pathological conditions.
© 2022 Zhu et al.
Figures
Similar articles
-
mTORC1 as the main gateway to autophagy.Essays Biochem. 2017 Dec 12;61(6):565-584. doi: 10.1042/EBC20170027. Print 2017 Dec 12. Essays Biochem. 2017. PMID: 29233869 Free PMC article. Review.
-
Molecular logic of mTORC1 signalling as a metabolic rheostat.Nat Metab. 2019 Mar;1(3):321-333. doi: 10.1038/s42255-019-0038-7. Epub 2019 Mar 4. Nat Metab. 2019. PMID: 32694720 Review.
-
Ferroptosis Regulation by Nutrient Signalling.Nutr Res Rev. 2022 Dec;35(2):282-294. doi: 10.1017/S0954422421000226. Epub 2021 Jul 8. Nutr Res Rev. 2022. PMID: 34233775 Review.
-
TFEB-driven endocytosis coordinates MTORC1 signaling and autophagy.Autophagy. 2019 Jan;15(1):151-164. doi: 10.1080/15548627.2018.1511504. Epub 2018 Sep 10. Autophagy. 2019. PMID: 30145926 Free PMC article.
-
Exosomal thioredoxin-1 from hypoxic human umbilical cord mesenchymal stem cells inhibits ferroptosis in doxorubicin-induced cardiotoxicity via mTORC1 signaling.Free Radic Biol Med. 2022 Nov 20;193(Pt 1):108-121. doi: 10.1016/j.freeradbiomed.2022.10.268. Epub 2022 Oct 12. Free Radic Biol Med. 2022. PMID: 36241072
Cited by
-
Psychedelics for acquired brain injury: a review of molecular mechanisms and therapeutic potential.Mol Psychiatry. 2024 Mar;29(3):671-685. doi: 10.1038/s41380-023-02360-0. Epub 2024 Jan 4. Mol Psychiatry. 2024. PMID: 38177350 Review.
-
Arginine alleviates Clostridium perfringens α toxin-induced intestinal injury in vivo and in vitro via the SLC38A9/mTORC1 pathway.Front Immunol. 2024 Apr 4;15:1357072. doi: 10.3389/fimmu.2024.1357072. eCollection 2024. Front Immunol. 2024. PMID: 38638435 Free PMC article.
-
Ferroptosis crosstalk in anti-tumor immunotherapy: molecular mechanisms, tumor microenvironment, application prospects.Apoptosis. 2024 Dec;29(11-12):1914-1943. doi: 10.1007/s10495-024-01997-8. Epub 2024 Jul 15. Apoptosis. 2024. PMID: 39008197 Review.
-
Extracellular Matrix Cues Regulate Mechanosensing and Mechanotransduction of Cancer Cells.Cells. 2024 Jan 2;13(1):96. doi: 10.3390/cells13010096. Cells. 2024. PMID: 38201302 Free PMC article. Review.
-
Role of FLCN Phosphorylation in Insulin-Mediated mTORC1 Activation and Tumorigenesis.Adv Sci (Weinh). 2023 Jun;10(17):e2206826. doi: 10.1002/advs.202206826. Epub 2023 Apr 21. Adv Sci (Weinh). 2023. PMID: 37083230 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

