A phosphine-based redox method for direct conjugation of disulfides
- PMID: 36278800
- PMCID: PMC9661873
- DOI: 10.1039/d2cc04967h
A phosphine-based redox method for direct conjugation of disulfides
Abstract
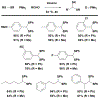
Technologies for cysteine disulfide detection and conjugation are pivotal to understanding protein functions and developing disulfide-derived therapeutic agents. Currently, disulfide modification requires reductive cleavage prior to functionalization, posing challenges to differentiating disulfides from free thiols. We describe herein Redox-assisted Disulfide Direct Conjugation (RDDC) as a new method to enable disulfide rebridging without cross-reacting with free thiols.
Conflict of interest statement
Conflicts of interest
The authors declare no conflicts of interest.
Figures
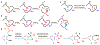
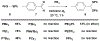
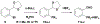
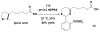
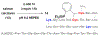
Similar articles
-
Automated high performance liquid chromatography with on-line reduction of disulfides and chemiluminescence detection for determination of thiols and disulfides in biological fluids.Anal Chim Acta. 2013 Mar 20;768:96-101. doi: 10.1016/j.aca.2013.01.035. Epub 2013 Jan 25. Anal Chim Acta. 2013. PMID: 23473255
-
Tris(3-hydroxypropyl)phosphine (THPP): A mild, air-stable reagent for the rapid, reductive cleavage of small-molecule disulfides.Bioorg Med Chem Lett. 2015 Oct 1;25(19):4114-7. doi: 10.1016/j.bmcl.2015.08.027. Epub 2015 Aug 14. Bioorg Med Chem Lett. 2015. PMID: 26318995
-
Quantification of thiols and disulfides.Biochim Biophys Acta. 2014 Feb;1840(2):838-46. doi: 10.1016/j.bbagen.2013.03.031. Epub 2013 Apr 6. Biochim Biophys Acta. 2014. PMID: 23567800 Free PMC article. Review.
-
Cyclic Thiosulfinates and Cyclic Disulfides Selectively Cross-Link Thiols While Avoiding Modification of Lone Thiols.J Am Chem Soc. 2018 Jun 20;140(24):7377-7380. doi: 10.1021/jacs.8b01136. Epub 2018 Jun 11. J Am Chem Soc. 2018. PMID: 29851341
-
Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation.Antioxid Redox Signal. 2005 Jul-Aug;7(7-8):964-72. doi: 10.1089/ars.2005.7.964. Antioxid Redox Signal. 2005. PMID: 15998251 Review.
Cited by
-
Naked-Eye Thiol Analyte Detection via Self-Propagating, Amplified Reaction Cycle.J Am Chem Soc. 2023 Oct 4;145(39):21222-21230. doi: 10.1021/jacs.3c02937. Epub 2023 Sep 25. J Am Chem Soc. 2023. PMID: 37748772 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

