Antimicrobial peptides for bone tissue engineering: Diversity, effects and applications
- PMID: 36277377
- PMCID: PMC9582762
- DOI: 10.3389/fbioe.2022.1030162
Antimicrobial peptides for bone tissue engineering: Diversity, effects and applications
Abstract
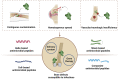
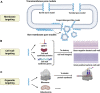
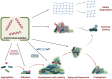
Bone tissue engineering has been becoming a promising strategy for surgical bone repair, but the risk of infection during trauma repair remains a problematic health concern worldwide, especially for fracture and infection-caused bone defects. Conventional antibiotics fail to effectively prevent or treat bone infections during bone defect repair because of drug-resistance and recurrence, so novel antibacterial agents with limited resistance are highly needed for bone tissue engineering. Antimicrobial peptides (AMPs) characterized by cationic, hydrophobic and amphipathic properties show great promise to be used as next-generation antibiotics which rarely induce resistance and show potent antibacterial efficacy. In this review, four common structures of AMPs (helix-based, sheet-based, coil-based and composite) and related modifications are presented to identify AMPs and design novel analogs. Then, potential effects of AMPs for bone infection during bone repair are explored, including bactericidal activity, anti-biofilm, immunomodulation and regenerative properties. Moreover, we present distinctive applications of AMPs for topical bone repair, which can be either used by delivery system (surface immobilization, nanoparticles and hydrogels) or used in gene therapy. Finally, future prospects and ongoing challenges are discussed.
Keywords: antimicrobial peptides; bone regeneration; delivery system; gene therapy; topical applications.
Copyright © 2022 Hao, Chen, Chai, Wang, Chen, Li, Hu, Feng and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects.Acta Biomater. 2020 Feb;103:52-67. doi: 10.1016/j.actbio.2019.12.025. Epub 2019 Dec 23. Acta Biomater. 2020. PMID: 31874224 Review.
-
Application of Antimicrobial Peptides of the Innate Immune System in Combination With Conventional Antibiotics-A Novel Way to Combat Antibiotic Resistance?Front Cell Infect Microbiol. 2019 Apr 30;9:128. doi: 10.3389/fcimb.2019.00128. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31114762 Free PMC article.
-
Antimicrobial Peptides and Macromolecules for Combating Microbial Infections: From Agents to Interfaces.ACS Appl Bio Mater. 2022 Feb 21;5(2):366-393. doi: 10.1021/acsabm.1c01132. Epub 2022 Jan 24. ACS Appl Bio Mater. 2022. PMID: 35072444 Review.
-
Antimicrobial peptides, conventional antibiotics, and their synergistic utility for the treatment of drug-resistant infections.Med Res Rev. 2022 Jul;42(4):1377-1422. doi: 10.1002/med.21879. Epub 2022 Jan 4. Med Res Rev. 2022. PMID: 34984699 Review.
-
Antibacterial Properties and Efficacy of a Novel SPLUNC1-Derived Antimicrobial Peptide, α4-Short, in a Murine Model of Respiratory Infection.mBio. 2019 Apr 9;10(2):e00226-19. doi: 10.1128/mBio.00226-19. mBio. 2019. PMID: 30967458 Free PMC article.
Cited by
-
Multiparametric influence of 3D-printed organo-mineral scaffolds on bone regeneration.Sci Rep. 2024 Sep 6;14(1):20848. doi: 10.1038/s41598-024-71698-4. Sci Rep. 2024. PMID: 39242756 Free PMC article.
-
Osteoporotic osseointegration: therapeutic hallmarks and engineering strategies.Theranostics. 2024 Jun 17;14(10):3859-3899. doi: 10.7150/thno.96516. eCollection 2024. Theranostics. 2024. PMID: 38994021 Free PMC article. Review.
-
Study of alloferon, a novel immunomodulatory antimicrobial peptide (AMP), and its analogues.Front Pharmacol. 2024 Feb 16;15:1359261. doi: 10.3389/fphar.2024.1359261. eCollection 2024. Front Pharmacol. 2024. PMID: 38434708 Free PMC article. Review.
-
Peptide-Based Biomaterials for Bone and Cartilage Regeneration.Biomedicines. 2024 Jan 29;12(2):313. doi: 10.3390/biomedicines12020313. Biomedicines. 2024. PMID: 38397915 Free PMC article. Review.
-
Shaping the Future of Antimicrobial Therapy: Harnessing the Power of Antimicrobial Peptides in Biomedical Applications.J Funct Biomater. 2023 Nov 2;14(11):539. doi: 10.3390/jfb14110539. J Funct Biomater. 2023. PMID: 37998108 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

