Epstein-Barr virus, interleukin-10 and multiple sclerosis: A ménage à trois
- PMID: 36275700
- PMCID: PMC9585213
- DOI: 10.3389/fimmu.2022.1028972
Epstein-Barr virus, interleukin-10 and multiple sclerosis: A ménage à trois
Abstract
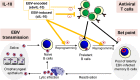
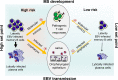
Multiple Sclerosis (MS) is an autoimmune disease that is characterized by inflammation and demyelination of nerve cells. There is strong evidence that Epstein-Barr virus (EBV), a human herpesvirus infecting B cells, greatly increases the risk of subsequent MS. Intriguingly, EBV not only induces human interleukin-10 but also encodes a homologue of this molecule, which is a key anti-inflammatory cytokine of the immune system. Although EBV-encoded IL-10 (ebvIL-10) has a high amino acid identity with its cellular counterpart (cIL-10), it shows more restricted and partially weaker functionality. We propose that both EBV-induced cIL-10 and ebvIL-10 act in a temporally and functionally coordinated manner helping the pathogen to establish latency in B cells and, at the same time, to balance the function of antiviral T cells. As a result, the EBV load persisting in the immune system is kept at a constant but individually different level (set point). During this immunological tug of war between virus and host, however, MS can be induced as collateral damage if the set point is too high. Here, we discuss a possible role of ebvIL-10 and EBV-induced cIL-10 in EBV-driven pathogenesis of MS.
Keywords: IL-10; antiviral immune responses; multiple sclerosis; viral IL-10; viral immune evasion; virus-induced immunopathogenesis; viruses.
Copyright © 2022 Schönrich, Abdelaziz and Raftery.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cumulative Roles for Epstein-Barr Virus, Human Endogenous Retroviruses, and Human Herpes Virus-6 in Driving an Inflammatory Cascade Underlying MS Pathogenesis.Front Immunol. 2021 Nov 1;12:757302. doi: 10.3389/fimmu.2021.757302. eCollection 2021. Front Immunol. 2021. PMID: 34790199 Free PMC article.
-
Epstein-Barr Virus-Specific CD8 T Cells Selectively Infiltrate the Brain in Multiple Sclerosis and Interact Locally with Virus-Infected Cells: Clue for a Virus-Driven Immunopathological Mechanism.J Virol. 2019 Nov 26;93(24):e00980-19. doi: 10.1128/JVI.00980-19. Print 2019 Dec 15. J Virol. 2019. PMID: 31578295 Free PMC article.
-
Antibody producing B lineage cells invade the central nervous system predominantly at the time of and triggered by acute Epstein-Barr virus infection: A hypothesis on the origin of intrathecal immunoglobulin synthesis in multiple sclerosis.Med Hypotheses. 2016 Jun;91:109-113. doi: 10.1016/j.mehy.2016.04.025. Epub 2016 Apr 16. Med Hypotheses. 2016. PMID: 27142157
-
Epstein-Barr virus and multiple sclerosis.Curr Neurol Neurosci Rep. 2007 May;7(3):253-8. doi: 10.1007/s11910-007-0038-y. Curr Neurol Neurosci Rep. 2007. PMID: 17488592 Review.
-
MINI-review of Epstein-Barr virus involvement in multiple sclerosis etiology and pathogenesis.J Neuroimmunol. 2022 Oct 15;371:577935. doi: 10.1016/j.jneuroim.2022.577935. Epub 2022 Jul 28. J Neuroimmunol. 2022. PMID: 35931008 Review.
Cited by
-
Editorial: Immune evasion mechanisms and their role in the pathogenesis of autoimmune disorders.Front Immunol. 2023 Sep 13;14:1267922. doi: 10.3389/fimmu.2023.1267922. eCollection 2023. Front Immunol. 2023. PMID: 37781356 Free PMC article. No abstract available.
-
Fibrinaloid Microclots and Atrial Fibrillation.Biomedicines. 2024 Apr 17;12(4):891. doi: 10.3390/biomedicines12040891. Biomedicines. 2024. PMID: 38672245 Free PMC article. Review.
-
Single-Cell Sequencing Combined with Transcriptome Sequencing Constructs a Predictive Model of Key Genes in Multiple Sclerosis and Explores Molecular Mechanisms Related to Cellular Communication.J Inflamm Res. 2024 Jan 9;17:191-210. doi: 10.2147/JIR.S442684. eCollection 2024. J Inflamm Res. 2024. PMID: 38226354 Free PMC article.
-
The Role of Selected Interleukins in the Development and Progression of Multiple Sclerosis-A Systematic Review.Int J Mol Sci. 2024 Feb 23;25(5):2589. doi: 10.3390/ijms25052589. Int J Mol Sci. 2024. PMID: 38473835 Free PMC article. Review.
-
Deciphering the Role of Epstein-Barr Virus Latent Membrane Protein 1 in Immune Modulation: A Multifaced Signalling Perspective.Viruses. 2024 Apr 4;16(4):564. doi: 10.3390/v16040564. Viruses. 2024. PMID: 38675906 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

