Human cutaneous interfollicular melanocytes differentiate temporarily under genotoxic stress
- PMID: 36274944
- PMCID: PMC9579029
- DOI: 10.1016/j.isci.2022.105238
Human cutaneous interfollicular melanocytes differentiate temporarily under genotoxic stress
Abstract
DNA-damage response of cutaneous interfollicular melanocytes to fractionated radiotherapy was investigated by immunostaining of tissue sections from punch biopsies collected before, during, and after the treatment of patients for breast cancer. Our clinical assay with sterilized hair follicles, excluded the migration of immature melanocytes from the bulge, and highlighted interfollicular melanocytes as an autonomous self-renewing population. About thirty percent are immature. Surrounding keratinocytes induced and maintained melanocyte differentiation as long as treatment was ongoing. Concomitant with differentiation, melanocytes were protected from apoptosis by transient upregulation of Bcl-2 and CXCR2. CXCR2 upregulation also indicated the instigation of premature senescence, preventing proliferation. The stem cell factor BMI1 was constitutively expressed exclusively in interfollicular melanocytes and further upregulated upon irradiation. BMI1 prevents apoptosis, terminal differentiation, and premature senescence, allowing dedifferentiation post-treatment, by suppressing the p53/p21-and p16-mediated response and upregulating CXCR2 to genotoxic damage. The pre-treatment immature subset of interfollicular melanocytes was restored after the exposure ended.
Keywords: Cancer; Cell biology; Developmental biology; Stem cells research.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
 ) cells per millimeter in the basal layer of epidermis (n = 15 patients). Error bars represent SEM. The dashed line represents the average of the data points.
) cells per millimeter in the basal layer of epidermis (n = 15 patients). Error bars represent SEM. The dashed line represents the average of the data points.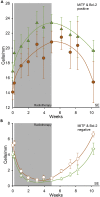
 ) and Bcl-2-positive melanocytes (
) and Bcl-2-positive melanocytes ( ). (B) MITF-negative cells (
). (B) MITF-negative cells ( ) and Bcl-2-negative cells (
) and Bcl-2-negative cells ( ) are morphologically characterized as melanocytes. Error bars represent SEM.
) are morphologically characterized as melanocytes. Error bars represent SEM.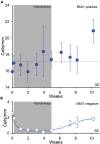
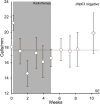
 ) and (B) BMI1-negative cells morphologically characterized as melanocytes (
) and (B) BMI1-negative cells morphologically characterized as melanocytes ( ). Error bars represent SEM. The dashed line represents the average of the data points.
). Error bars represent SEM. The dashed line represents the average of the data points.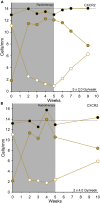
 ) and CXCR2-negative cells (
) and CXCR2-negative cells ( ) were morphologically characterized as melanocytes, and the total number of stained and unstained melanocytes is indicated (
) were morphologically characterized as melanocytes, and the total number of stained and unstained melanocytes is indicated ( ). Reference line represents the mean number of melanocytes counted in eosin-PAS staining (Turesson et al., 2020).
). Reference line represents the mean number of melanocytes counted in eosin-PAS staining (Turesson et al., 2020).
 ).
).
Similar articles
-
UV-Radiation Response Proteins Reveal Undifferentiated Cutaneous Interfollicular Melanocytes with Hyperradiosensitivity to Differentiation at 0.05 Gy Radiotherapy Dose Fractions.Radiat Res. 2019 Jan;191(1):93-106. doi: 10.1667/RR15078.1. Epub 2018 Nov 8. Radiat Res. 2019. PMID: 30407899
-
Epidermal Keratinocyte Depletion during Five Weeks of Radiotherapy is Associated with DNA Double-Strand Break Foci, Cell Growth Arrest and Apoptosis: Evidence of Increasing Radioresponsiveness and Lack of Repopulation; the Number of Melanocytes Remains Unchanged.Radiat Res. 2020 May;193(5):481-496. doi: 10.1667/RR15417.1. Epub 2020 Mar 20. Radiat Res. 2020. PMID: 32196412
-
Repigmentation through Melanocyte Regeneration in Vitiligo.Dermatol Clin. 2017 Apr;35(2):205-218. doi: 10.1016/j.det.2016.11.015. Dermatol Clin. 2017. PMID: 28317529 Review.
-
Maintenance of distinct melanocyte populations in the interfollicular epidermis.Pigment Cell Melanoma Res. 2015 Jul;28(4):476-80. doi: 10.1111/pcmr.12375. Epub 2015 Apr 30. Pigment Cell Melanoma Res. 2015. PMID: 25847135 Free PMC article.
-
Melanocyte stem cells: biology and current aspects.Med Sci Monit. 2012 Oct;18(10):RA155-9. doi: 10.12659/msm.883475. Med Sci Monit. 2012. PMID: 23018363 Free PMC article. Review.
Cited by
-
Increased Peripheral Blood DNA Damage and Elevated Serum Levels of Melanoma Inhibitory Activity Protein: Clues to Excess Skin Cancer Risk in Airline Pilots?Cureus. 2023 Dec 25;15(12):e51077. doi: 10.7759/cureus.51077. eCollection 2023 Dec. Cureus. 2023. PMID: 38269211 Free PMC article.
-
BMI1 is required for melanocyte stem cell maintenance and hair pigmentation.Pigment Cell Melanoma Res. 2023 Sep;36(5):399-406. doi: 10.1111/pcmr.13088. Epub 2023 May 3. Pigment Cell Melanoma Res. 2023. PMID: 37132544 Free PMC article.
References
-
- Acosta J.C., O'Loghlen A., Banito A., Guijarro M.V., Augert A., Raguz S., Fumagalli M., Da Costa M., Brown C., Popov N., et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
-
- Barlow J.O., Maize J., Sr., Lang P.G. The density and distribution of melanocytes adjacent to melanoma and nonmelanoma skin cancers. Dermatol. Surg. 2007;33:199–207. - PubMed
-
- Bellei B., Pitisci A., Catricalà C., Larue L., Picardo M. Wnt/beta-catenin signaling is stimulated by alpha-melanocyte-stimulating hormone in melanoma and melanocyte cells: implication in cell differentiation. Pigment Cell Melanoma Res. 2011;24:309–325. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

