Histone deacetylase inhibitors enhance oncolytic herpes simplex virus therapy for malignant meningioma
- PMID: 36271587
- PMCID: PMC9590235
- DOI: 10.1016/j.biopha.2022.113843
Histone deacetylase inhibitors enhance oncolytic herpes simplex virus therapy for malignant meningioma
Abstract
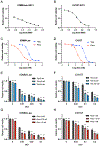
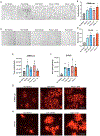
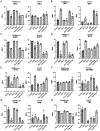
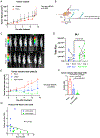
Approximately 20% of meningiomas are not benign (higher grade) and tend to relapse after surgery and radiation therapy. Malignant (anaplastic) meningioma (MM) is a minor subset of high-grade meningioma that is lethal with no effective treatment options currently. Oncolytic herpes simplex virus (oHSV) is a powerful anti-cancer modality that induces both direct cell death and anti-tumor immunity, and has shown activity in preclinical models of MM. However, clinically meaningful efficacy will likely entail rational mechanistic combination approaches. We here show that epigenome modulator histone deacetylase inhibitors (HDACi) increase anti-cancer effects of oHSV in human MM models, IOMM-Lee (NF2 wild-type) and CH157 (NF2 mutant). Minimally toxic, sub-micromolar concentrations of pan-HDACi, Trichostatin A and Panobinostat, substantively increased the infectability and spread of oHSV G47Δ within MM cells in vitro, resulting in enhanced oHSV-mediated killing of target cells when infected at low multiplicity of infection (MOI). Transcriptomics analysis identified selective alteration of mRNA processing and splicing modules that might underlie the potent anti-MM effects of combining HDACi and oHSV. In vivo, HDACi treatment increased intratumoral oHSV replication and boosted the capacity of oHSV to control the growth of human MM xenografts. Thus, our work supports further translational development of the combination approach employing HDACi and oHSV for the treatment of MM.
Keywords: Histone deacetylase inhibitor; Malignant meningioma; Meningioma; Oncolytic herpes simplex virus.
Copyright © 2022 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Conflict of interest statement SDR and RLM are co-inventors on patents relating to oncolytic herpes simplex viruses, owned and managed by Georgetown University and Massachusetts General Hospital, which have received royalties from Amgen and ActiVec Inc. SDR acted as a consultant and received honoraria from Replimune, Cellinta, and Greenfire Bio, and honoraria and equity from EG 427. RLM. is on the S.A.B. and receives payment from Virogin Biotech Ltd.
Figures

Similar articles
-
Histone Deacetylase Inhibitor Panobinostat Benefits the Therapeutic Efficacy of Oncolytic Herpes Simplex Virus Combined with PD-1/PD-L1 Blocking in Glioma and Squamous Cell Carcinoma Models.Viruses. 2022 Dec 15;14(12):2796. doi: 10.3390/v14122796. Viruses. 2022. PMID: 36560800 Free PMC article.
-
A new patient-derived orthotopic malignant meningioma model treated with oncolytic herpes simplex virus.Neuro Oncol. 2016 Sep;18(9):1278-87. doi: 10.1093/neuonc/now031. Epub 2016 Mar 6. Neuro Oncol. 2016. PMID: 26951380 Free PMC article.
-
Histone deacetylase inhibitors improve the replication of oncolytic herpes simplex virus in breast cancer cells.PLoS One. 2014 Mar 20;9(3):e92919. doi: 10.1371/journal.pone.0092919. eCollection 2014. PLoS One. 2014. PMID: 24651853 Free PMC article.
-
Oncolytic herpes simplex virus-based strategies: toward a breakthrough in glioblastoma therapy.Front Microbiol. 2014 Jun 20;5:303. doi: 10.3389/fmicb.2014.00303. eCollection 2014. Front Microbiol. 2014. PMID: 24999342 Free PMC article. Review.
-
Oncolytic Herpes Simplex Virus-Based Therapies for Cancer.Cells. 2021 Jun 18;10(6):1541. doi: 10.3390/cells10061541. Cells. 2021. PMID: 34207386 Free PMC article. Review.
Cited by
-
Histone deacetylase inhibitor boosts anticancer potential of fusogenic oncolytic vaccinia virus by enhancing cell-cell fusion.Cancer Sci. 2024 Feb;115(2):600-610. doi: 10.1111/cas.16032. Epub 2023 Nov 30. Cancer Sci. 2024. PMID: 38037288 Free PMC article.
-
Caspase 3 Expression Profiles in Meningioma Subtypes Based on Tissue Microarray Analysis.Cancer Diagn Progn. 2024 Sep 1;4(5):586-591. doi: 10.21873/cdp.10367. eCollection 2024 Sep-Oct. Cancer Diagn Progn. 2024. PMID: 39238614 Free PMC article.
-
Advances in antitumour therapy with oncolytic herpes simplex virus combinations.Discov Oncol. 2024 Jul 24;15(1):302. doi: 10.1007/s12672-024-01165-z. Discov Oncol. 2024. PMID: 39046631 Free PMC article. Review.
-
Development and application of oncolytic viruses as the nemesis of tumor cells.Front Microbiol. 2023 Jun 12;14:1188526. doi: 10.3389/fmicb.2023.1188526. eCollection 2023. Front Microbiol. 2023. PMID: 37440883 Free PMC article. Review.
-
Positioning SUMO as an immunological facilitator of oncolytic viruses for high-grade glioma.Front Cell Dev Biol. 2023 Oct 4;11:1271575. doi: 10.3389/fcell.2023.1271575. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37860820 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

