Biomimetic cytomembrane-coated ZIF-8-loaded DMDD nanoparticle and sonodynamic co-therapy for cancer
- PMID: 36267767
- PMCID: PMC9577741
- DOI: 10.21037/atm-22-3646
Biomimetic cytomembrane-coated ZIF-8-loaded DMDD nanoparticle and sonodynamic co-therapy for cancer
Abstract
Background: Breast cancer (BC) is the most common type of cancer affecting females. It is also a leading cause of cancer-related death in women worldwide.
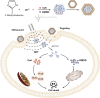
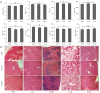
Methods: Sonodynamic therapy (SDT) is an emerging therapeutic strategy for cancer treatment. SDT ensures non-invasive penetration of deep tumors and results in activation of non-toxic sonosensitizers administered in deep tumor sites to become cytotoxic. It has been reported that 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione (DMDD) has a significant anti-tumor effect against various cancer types including BC. However, DMDD is hydrophobic. Therefore, a one-step encapsulation method was used in the current study to construct zeolitic imidazole frameworks-8 (ZIF-8) loaded with DMDD and sonosensitizer chlorin e6 (Ce6). ZIF-8 was further modified by coating it with a biomimetic cell membrane to improve targeted delivery.
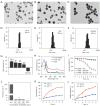
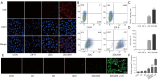
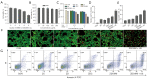
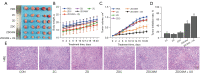
Results: In vitro and in vivo results indicated that the nanomedicines had great biocompatibility properties and targeting ability. The nanocomposite exhibited a higher release rate under an acidic tumor microenvironment. The tumor killing effect of reactive oxygen species (ROS) generated from Ce6 and inhibition of tumor growth was enhanced after ultrasound (US) treatment, which might be caused by the increase in apoptosis rate.
Conclusions: These findings show that the combination of nanomedicine and SDT provides a potential therapeutic method for BC.
Keywords: 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione (DMDD); Breast cancer (BC); ZIF-8 nanoparticles; metal-organic framework; sonodynamic therapy.
2022 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3646/coif). The authors have no conflicts of interest to declare.
Figures

Similar articles
-
A Novel Platform of MOF for Sonodynamic Therapy Advanced Therapies.Pharmaceutics. 2023 Aug 1;15(8):2071. doi: 10.3390/pharmaceutics15082071. Pharmaceutics. 2023. PMID: 37631285 Free PMC article. Review.
-
Biomimetic Metal-Organic Framework Nanoparticles for Synergistic Combining of SDT-Chemotherapy Induce Pyroptosis in Gastric Cancer.Front Bioeng Biotechnol. 2022 Feb 21;10:796820. doi: 10.3389/fbioe.2022.796820. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35265591 Free PMC article.
-
Mitochondria-targeting sonosensitizer-loaded extracellular vesicles for chemo-sonodynamic therapy.J Control Release. 2023 Feb;354:651-663. doi: 10.1016/j.jconrel.2023.01.044. Epub 2023 Jan 25. J Control Release. 2023. PMID: 36682729
-
Efficient Combination Chemo-Sonodynamic Cancer Therapy Using Mitochondria-Targeting Sonosensitizer-Loaded Polysorbate-Based Micelles.Int J Mol Sci. 2024 Mar 20;25(6):3474. doi: 10.3390/ijms25063474. Int J Mol Sci. 2024. PMID: 38542447 Free PMC article.
-
Inorganic chemoreactive nanosonosensitzers with unique physiochemical properties and structural features for versatile sonodynamic nanotherapies.Biomed Mater. 2021 Apr 21;16(3). doi: 10.1088/1748-605X/abef58. Biomed Mater. 2021. PMID: 33725684 Review.
Cited by
-
A Novel Platform of MOF for Sonodynamic Therapy Advanced Therapies.Pharmaceutics. 2023 Aug 1;15(8):2071. doi: 10.3390/pharmaceutics15082071. Pharmaceutics. 2023. PMID: 37631285 Free PMC article. Review.
-
Application of Nanomaterial-Based Sonodynamic Therapy in Tumor Therapy.Pharmaceutics. 2024 Apr 29;16(5):603. doi: 10.3390/pharmaceutics16050603. Pharmaceutics. 2024. PMID: 38794265 Free PMC article. Review.
-
A sono-responsive antibacterial nanosystem co-loaded with metformin and bone morphogenetic protein-2 for mitigation of inflammation and bone loss in experimental peri-implantitis.Front Bioeng Biotechnol. 2024 May 24;12:1410230. doi: 10.3389/fbioe.2024.1410230. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38854857 Free PMC article.
-
Biomimetic ZIF-8 Nanoparticles: A Novel Approach for Biomimetic Drug Delivery Systems.Int J Nanomedicine. 2024 Jun 10;19:5523-5544. doi: 10.2147/IJN.S462480. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38882544 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
