Comprehensive analysis of cuproptosis in immune response and prognosis of osteosarcoma
- PMID: 36263140
- PMCID: PMC9573992
- DOI: 10.3389/fphar.2022.992431
Comprehensive analysis of cuproptosis in immune response and prognosis of osteosarcoma
Abstract
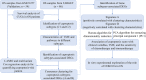
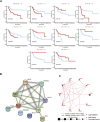
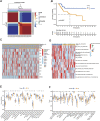
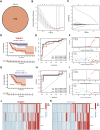
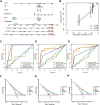
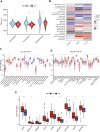
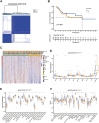
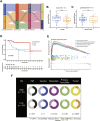
Copper-induced cell death, a form of apoptosis, has been extensively investigated in human diseases. Recent studies on the mechanisms underlying copper-induced cell death have provided innovative insights into copper-related toxicity in cells, and this form of programmed cell death was termed cuproptosis. Herein, we conducted a comprehensive analysis to determine the specific role of cuproptosis in osteosarcoma. Using consensus clustering analysis, patients with osteosarcoma from the TARGET database were classified into subgroups with distinct cuproptosis-based molecular patterns. Accordingly, these patients displayed diverse clinicopathological features, survival outcomes, tumor microenvironment (TME) characteristics, immune-related scores, and therapeutic responses. Furthermore, we constructed a cuproptosis-based risk signature and nomogram, as well as developed a cuproptosis score for improved patient characterization. The prognostic model and cuproptosis score were well validated and confirmed to efficiently distinguish high- and low-risk patients, thereby affording great predictive value. Finally, we verified the abnormal expression of prognostic CUG in OS patients by immunohistochemistry. In conclusion, we suggest that cuproptosis may play an important role in regulating the tumor microenvironment features, tumor progression and the long-term prognosis of osteosarcoma.
Keywords: copper-induced cell death; cuproptosis; immunotherapy; osteosarcoma; tumor microenvironment.
Copyright © 2022 Li, Song, Bai, Hua, Wu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Comprehensive investigation into cuproptosis in the characterization of clinical features, molecular characteristics, and immune situations of clear cell renal cell carcinoma.Front Immunol. 2022 Oct 6;13:948042. doi: 10.3389/fimmu.2022.948042. eCollection 2022. Front Immunol. 2022. PMID: 36275737 Free PMC article.
-
Comprehensive analysis of cuproptosis-related genes and tumor microenvironment infiltration characterization in breast cancer.Front Immunol. 2022 Oct 20;13:978909. doi: 10.3389/fimmu.2022.978909. eCollection 2022. Front Immunol. 2022. PMID: 36341328 Free PMC article.
-
Identification of a novel cuproptosis-related gene signature and integrative analyses in patients with lower-grade gliomas.Front Immunol. 2022 Aug 15;13:933973. doi: 10.3389/fimmu.2022.933973. eCollection 2022. Front Immunol. 2022. PMID: 36045691 Free PMC article. Review.
-
Cuproptosis-related modification patterns depict the tumor microenvironment, precision immunotherapy, and prognosis of kidney renal clear cell carcinoma.Front Immunol. 2022 Sep 23;13:933241. doi: 10.3389/fimmu.2022.933241. eCollection 2022. Front Immunol. 2022. PMID: 36211378 Free PMC article.
-
Roles and mechanisms of copper homeostasis and cuproptosis in osteoarticular diseases.Biomed Pharmacother. 2024 May;174:116570. doi: 10.1016/j.biopha.2024.116570. Epub 2024 Apr 9. Biomed Pharmacother. 2024. PMID: 38599063 Review.
Cited by
-
Multifunctional nanomaterials via cell cuproptosis and oxidative stress for treating osteosarcoma and OS-induced bone destruction.Mater Today Bio. 2024 Feb 15;25:100996. doi: 10.1016/j.mtbio.2024.100996. eCollection 2024 Apr. Mater Today Bio. 2024. PMID: 38420143 Free PMC article.
References
LinkOut - more resources
Full Text Sources

