Emerging roles for IL-25 and IL-33 in colorectal cancer tumorigenesis
- PMID: 36263033
- PMCID: PMC9573978
- DOI: 10.3389/fimmu.2022.981479
Emerging roles for IL-25 and IL-33 in colorectal cancer tumorigenesis
Abstract
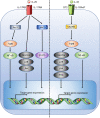
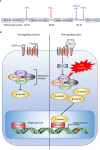
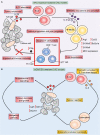
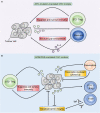
Colorectal cancer (CRC) is the second leading cause of cancer-related death worldwide, and is largely refractory to current immunotherapeutic interventions. The lack of efficacy of existing cancer immunotherapies in CRC reflects the complex nature of the unique intestinal immune environment, which serves to maintain barrier integrity against pathogens and harmful environmental stimuli while sustaining host-microbe symbiosis during homeostasis. With their expression by barrier epithelial cells, the cytokines interleukin-25 (IL-25) and IL-33 play key roles in intestinal immune responses, and have been associated with inappropriate allergic reactions, autoimmune diseases and cancer pathology. Studies in the past decade have begun to uncover the important roles of IL-25 and IL-33 in shaping the CRC tumour immune microenvironment, where they may promote or inhibit tumorigenesis depending on the specific CRC subtype. Notably, both IL-25 and IL-33 have been shown to act on group 2 innate lymphoid cells (ILC2s), but can also stimulate an array of other innate and adaptive immune cell types. Though sometimes their functions can overlap they can also produce distinct phenotypes dependent on the differential distribution of their receptor expression. Furthermore, both IL-25 and IL-33 modulate pathways previously known to contribute to CRC tumorigenesis, including angiogenesis, tumour stemness, invasion and metastasis. Here, we review our current understanding of IL-25 and IL-33 in CRC tumorigenesis, with specific focus on dissecting their individual function in the context of distinct subtypes of CRC, and the potential prospects for targeting these pathways in CRC immunotherapy.
Keywords: IL-25; IL-33; colorectal cancer; cytokine; microenvironment; mouse model.
Copyright © 2022 Jou, Rodriguez-Rodriguez and McKenzie.
Conflict of interest statement
ANJM is on the scientific advisory board of SinoMab. ANJM developed antibodies against IL-25R which MRC has licenced to SinoMab. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
An innate IL-25-ILC2-MDSC axis creates a cancer-permissive microenvironment for Apc mutation-driven intestinal tumorigenesis.Sci Immunol. 2022 Jun 3;7(72):eabn0175. doi: 10.1126/sciimmunol.abn0175. Epub 2022 Jun 3. Sci Immunol. 2022. PMID: 35658010 Free PMC article.
-
Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer.J Immunother Cancer. 2021 Jan;9(1):e001895. doi: 10.1136/jitc-2020-001895. J Immunother Cancer. 2021. PMID: 33462141 Free PMC article.
-
IL-33 promotes growth and liver metastasis of colorectal cancer in mice by remodeling the tumor microenvironment and inducing angiogenesis.Mol Carcinog. 2017 Jan;56(1):272-287. doi: 10.1002/mc.22491. Epub 2016 Apr 27. Mol Carcinog. 2017. PMID: 27120577 Free PMC article.
-
Interleukin-33 in tumorigenesis, tumor immune evasion, and cancer immunotherapy.J Mol Med (Berl). 2016 May;94(5):535-43. doi: 10.1007/s00109-016-1397-0. Epub 2016 Feb 27. J Mol Med (Berl). 2016. PMID: 26922618 Review.
-
Metabolism and Colorectal Cancer.Annu Rev Pathol. 2023 Jan 24;18:467-492. doi: 10.1146/annurev-pathmechdis-031521-041113. Epub 2022 Nov 2. Annu Rev Pathol. 2023. PMID: 36323004 Free PMC article. Review.
Cited by
-
Colorectal Cancer in Correlation With Clinicopathological Variables: The Effects of Hypoxia-Inducible Factor-1 Alfa or the InterLeukin-33 and Vascular Endothelial Growth Factor?Cureus. 2024 Jan 4;16(1):e51658. doi: 10.7759/cureus.51658. eCollection 2024 Jan. Cureus. 2024. PMID: 38313904 Free PMC article.
-
Serum Interleukins 8, 17, and 33 as Potential Biomarkers of Colon Cancer.Cancers (Basel). 2024 Feb 10;16(4):745. doi: 10.3390/cancers16040745. Cancers (Basel). 2024. PMID: 38398137 Free PMC article.
-
Type 1 and type 2 cytokine-mediated immune orchestration in the tumour microenvironment and their therapeutic potential.Explor Target Antitumor Ther. 2023;4(3):474-497. doi: 10.37349/etat.2023.00146. Epub 2023 Jun 30. Explor Target Antitumor Ther. 2023. PMID: 37455828 Free PMC article. Review.
-
Regenerating gene 4 promotes chemoresistance of colorectal cancer by affecting lipid droplet synthesis and assembly.World J Gastroenterol. 2023 Sep 21;29(35):5104-5124. doi: 10.3748/wjg.v29.i35.5104. World J Gastroenterol. 2023. PMID: 37744296 Free PMC article.
-
Novel therapeutic strategies targeting myeloid-derived suppressor cell immunosuppressive mechanisms for cancer treatment.Explor Target Antitumor Ther. 2024;5(1):187-207. doi: 10.37349/etat.2024.00212. Epub 2024 Feb 28. Explor Target Antitumor Ther. 2024. PMID: 38464388 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

