scRNA-seq generates a molecular map of emerging cell subtypes after sciatic nerve injury in rats
- PMID: 36261573
- PMCID: PMC9581950
- DOI: 10.1038/s42003-022-03970-0
scRNA-seq generates a molecular map of emerging cell subtypes after sciatic nerve injury in rats
Abstract
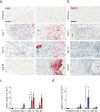
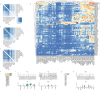
Patients with peripheral nerve injury, viral infection or metabolic disorder often suffer neuropathic pain due to inadequate pharmacological options for relief. Developing novel therapies has been challenged by incomplete mechanistic understanding of the cellular microenvironment in sensory nerve that trigger the emergence and persistence of pain. In this study, we report a high resolution transcriptomics map of the cellular heterogeneity of naïve and injured rat sensory nerve covering more than 110,000 individual cells. Annotation reveals distinguishing molecular features of multiple major cell types totaling 45 different subtypes in naïve nerve and an additional 23 subtypes emerging after injury. Ligand-receptor analysis revealed a myriad of potential targets for pharmacological intervention. This work forms a comprehensive resource and unprecedented window into the cellular milieu underlying neuropathic pain and demonstrates that nerve injury is a dynamic process orchestrated by multiple cell types in both the endoneurial and epineurial nerve compartments.
© 2022. Merck & Co., Inc., Rahway, NJ, USA and its affiliates.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Effects of miR-150 on neuropathic pain process via targeting AKT3.Biochem Biophys Res Commun. 2019 Sep 24;517(3):532-537. doi: 10.1016/j.bbrc.2019.07.061. Epub 2019 Jul 31. Biochem Biophys Res Commun. 2019. PMID: 31376943
-
Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents.Mol Pain. 2016 May 12;12:1744806916646784. doi: 10.1177/1744806916646784. Print 2016. Mol Pain. 2016. PMID: 27175012 Free PMC article.
-
Assessment of sensory thresholds and nociceptive fiber growth after sciatic nerve injury reveals the differential contribution of collateral reinnervation and nerve regeneration to neuropathic pain.Exp Neurol. 2014 May;255:1-11. doi: 10.1016/j.expneurol.2014.02.008. Epub 2014 Feb 16. Exp Neurol. 2014. PMID: 24552688
-
Spinal P2X(7) receptor mediates microglia activation-induced neuropathic pain in the sciatic nerve injury rat model.Behav Brain Res. 2012 Jan 1;226(1):163-70. doi: 10.1016/j.bbr.2011.09.015. Epub 2011 Sep 14. Behav Brain Res. 2012. PMID: 21933684
-
Lack of interleukin-17 leads to a modulated micro-environment and amelioration of mechanical hypersensitivity after peripheral nerve injury in mice.Pain. 2014 Jul;155(7):1293-1302. doi: 10.1016/j.pain.2014.04.004. Epub 2014 Apr 8. Pain. 2014. PMID: 24721689
Cited by
-
Single-cell RNA sequencing of neurofibromas reveals a tumor microenvironment favorable for neural regeneration and immune suppression in a neurofibromatosis type 1 porcine model.Front Oncol. 2023 Sep 25;13:1253659. doi: 10.3389/fonc.2023.1253659. eCollection 2023. Front Oncol. 2023. PMID: 37817770 Free PMC article.
-
Local Administration of Minocycline Improves Nerve Regeneration in Two Rat Nerve Injury Models.Int J Mol Sci. 2023 Jul 28;24(15):12085. doi: 10.3390/ijms241512085. Int J Mol Sci. 2023. PMID: 37569473 Free PMC article.
-
Single-cell transcriptome analysis reveals distinct cell populations in dorsal root ganglia and their potential roles in diabetic peripheral neuropathy.PLoS One. 2024 Jul 31;19(7):e0306424. doi: 10.1371/journal.pone.0306424. eCollection 2024. PLoS One. 2024. PMID: 39083491 Free PMC article.
-
Stellate cells are in utero markers of pancreatic disease in cystic fibrosis.Mol Med. 2024 Aug 7;30(1):115. doi: 10.1186/s10020-024-00871-2. Mol Med. 2024. PMID: 39112965 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

