Decursinol-mediated antinociception and anti-allodynia in acute and neuropathic pain models in male mice: Tolerance and receptor profiling
- PMID: 36249788
- PMCID: PMC9558739
- DOI: 10.3389/fphar.2022.968976
Decursinol-mediated antinociception and anti-allodynia in acute and neuropathic pain models in male mice: Tolerance and receptor profiling
Abstract
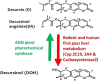
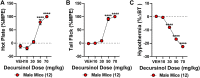
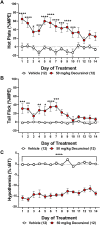
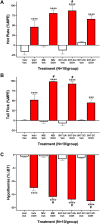
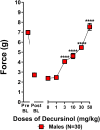
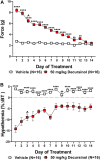
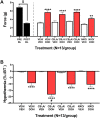
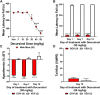
Korean scientists have shown that oral administration of Angelica gigas Nakai (AGN) root alcoholic extract and the metabolite of its pyranocoumarins, decursinol, have antinociceptive properties across various thermal and acute inflammatory pain models. The objectives of this study were 1) to assess whether tolerance develops to the antinociceptive effects of once-daily intraperitoneally administered decursinol (50 mg/kg) in acute thermal pain models, 2) to establish its anti-allodynic efficacy and potential tolerance development in a model of chemotherapy-evoked neuropathic pain (CENP) and 3) to probe the involvement of select receptors in mediating the pain-relieving effects with antagonists. The results show that decursinol induced antinociception in both the hot plate and tail-flick assays and reversed mechanical allodynia in mice with cisplatin-evoked neuropathic pain. Tolerance was detected to the antinociceptive effects of decursinol in the hot plate and tail-flick assays and to the anti-allodynic effects of decursinol in neuropathic mice. Pretreatment with either the 5-HT2 antagonist methysergide, the 5-HT2A antagonist volinanserin, or the 5-HT2C antagonist SB-242084 failed to attenuate decursinol-induced antinociception in the tail-flick assay. While pretreatment with the cannabinoid inverse agonists rimonabant and SR144528 failed to modify decursinol-induced anti-allodynia, pretreatment with the opioid antagonist naloxone partially attenuated the anti-allodynic effects of decursinol. In conclusion, our data support decursinol as an active phytochemical of AGN having both antinociceptive and anti-allodynic properties. Future work warrants a more critical investigation of potential receptor mechanisms as they are likely more complicated than initially reported.
Keywords: antinociception; decursinol; mice; neuropathic pain; tolerance.
Copyright © 2022 Crawford, Kim, Karelia, Sepulveda, Morgan, Lü and Henderson-Redmond.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Antinociceptive mechanisms of orally administered decursinol in the mouse.Life Sci. 2003 Jun 13;73(4):471-85. doi: 10.1016/s0024-3205(03)00311-4. Life Sci. 2003. PMID: 12759141
-
Tolerance to the antinociceptive effects of chronic morphine requires c-Jun N-terminal kinase.Mol Pain. 2015 Jun 12;11:34. doi: 10.1186/s12990-015-0031-4. Mol Pain. 2015. PMID: 26065412 Free PMC article.
-
The spinal anti-allodynic effects of endomorphin analogs with C-terminal hydrazide modification in neuropathic pain model.Peptides. 2020 Dec;134:170407. doi: 10.1016/j.peptides.2020.170407. Epub 2020 Sep 11. Peptides. 2020. PMID: 32926948
-
Anti-cancer and other bioactivities of Korean Angelica gigas Nakai (AGN) and its major pyranocoumarin compounds.Anticancer Agents Med Chem. 2012 Dec;12(10):1239-54. doi: 10.2174/187152012803833071. Anticancer Agents Med Chem. 2012. PMID: 22583405 Review.
-
Anticancer potential of decursin, decursinol angelate, and decursinol from Angelica gigas Nakai: A comprehensive review and future therapeutic prospects.Food Sci Nutr. 2024 Jul 31;12(10):6970-6989. doi: 10.1002/fsn3.4376. eCollection 2024 Oct. Food Sci Nutr. 2024. PMID: 39479643 Free PMC article. Review.
References
-
- Ahn K-S., Woong-Seop SimKim I-H. (1995). Detection of anticancer activity from the root of Angelica gigas in vitro . J. Microbiol. Biotechnol., 5, 105–109. Available at: https://www.koreascience.or.kr/article/JAKO199511922233256.page (Accessed Apr 28, 2022).
Grants and funding
LinkOut - more resources
Full Text Sources

