BNTA alleviates inflammatory osteolysis by the SOD mediated anti-oxidation and anti-inflammation effect on inhibiting osteoclastogenesis
- PMID: 36249770
- PMCID: PMC9559729
- DOI: 10.3389/fphar.2022.939929
BNTA alleviates inflammatory osteolysis by the SOD mediated anti-oxidation and anti-inflammation effect on inhibiting osteoclastogenesis
Abstract
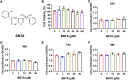
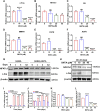
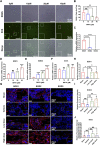
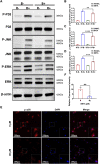
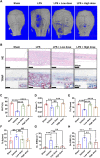
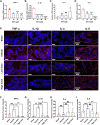
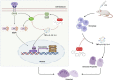
Abnormal activation and overproliferation of osteoclast in inflammatory bone diseases lead to osteolysis and bone mass loss. Although current pharmacological treatments have made extensive advances, limitations still exist. N-[2-bromo-4-(phenylsulfonyl)-3-thienyl]-2-chlorobenzamide (BNTA) is an artificially synthesized molecule compound that has antioxidant and anti-inflammatory properties. In this study, we presented that BNTA can suppress intracellular ROS levels through increasing ROS scavenging enzymes SOD1 and SOD2, subsequently attenuating the MARK signaling pathway and the transcription of NFATc1, leading to the inhibition of osteoclast formation and osteolytic resorption. Moreover, the results also showed an obvious restrained effect of BNTA on RANKL-stimulated proinflammatory cytokines, which indirectly mediated osteoclastogenesis. In line with the in vitro results, BNTA protected LPS-induced severe bone loss in vivo by enhancing scavenging enzymes, reducing proinflammatory cytokines, and decreasing osteoclast formation. Taken together, all of the results demonstrate that BNTA effectively represses oxidation, regulates inflammatory activity, and inhibits osteolytic bone resorption, and it may be a potential and exploitable drug to prevent inflammatory osteolytic bone diseases.
Keywords: BNTA; MAPK; SOD; bone loss; inflammatory osteolysis; osteolysis.
Copyright © 2022 Wang, Cao, Guo, Yang, Sun, Fu, Qin, Wu and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Diallyl disulfide alleviates inflammatory osteolysis by suppressing osteoclastogenesis via NF-κB-NFATc1 signal pathway.FASEB J. 2019 Jun;33(6):7261-7273. doi: 10.1096/fj.201802172R. Epub 2019 Mar 11. FASEB J. 2019. PMID: 30857415 Free PMC article.
-
Luteoloside prevents lipopolysaccharide-induced osteolysis and suppresses RANKL-induced osteoclastogenesis through attenuating RANKL signaling cascades.J Cell Physiol. 2018 Feb;233(2):1723-1735. doi: 10.1002/jcp.26084. Epub 2017 Aug 23. J Cell Physiol. 2018. PMID: 28681916
-
Acacetin inhibits RANKL-induced osteoclastogenesis and LPS-induced bone loss by modulating NFATc1 transcription.Biochem Biophys Res Commun. 2021 Oct 30;583:146-153. doi: 10.1016/j.bbrc.2021.10.066. Online ahead of print. Biochem Biophys Res Commun. 2021. PMID: 34763194
-
Interleukin-27 prevents LPS-induced inflammatory osteolysis by inhibiting osteoclast formation and function.Am J Transl Res. 2019 Mar 15;11(3):1154-1169. eCollection 2019. Am J Transl Res. 2019. PMID: 30972153 Free PMC article. Review.
-
The Function and Mechanism of Anti-Inflammatory Factor Metrnl Prevents the Progression of Inflammatory-Mediated Pathological Bone Osteolytic Diseases.J Inflamm Res. 2024 Mar 11;17:1607-1619. doi: 10.2147/JIR.S455790. eCollection 2024. J Inflamm Res. 2024. PMID: 38495340 Free PMC article. Review.
Cited by
-
Pyroptosis in Periprosthetic Osteolysis.Biomolecules. 2022 Nov 23;12(12):1733. doi: 10.3390/biom12121733. Biomolecules. 2022. PMID: 36551161 Free PMC article. Review.
-
Gut microbiota and metabolic profile changes unveil the deterioration of alveolar bone inflammatory resorption with aging induced by D-galactose.Sci Rep. 2024 Oct 30;14(1):26135. doi: 10.1038/s41598-024-75941-w. Sci Rep. 2024. PMID: 39477973 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

