Sterile protection and transmission blockade by a multistage anti-malarial vaccine in the pre-clinical study
- PMID: 36248835
- PMCID: PMC9558734
- DOI: 10.3389/fimmu.2022.1005476
Sterile protection and transmission blockade by a multistage anti-malarial vaccine in the pre-clinical study
Abstract
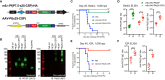
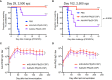
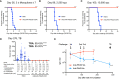
The Malaria Vaccine Technology Roadmap 2013 (World Health Organization) aims to develop safe and effective vaccines by 2030 that will offer at least 75% protective efficacy against clinical malaria and reduce parasite transmission. Here, we demonstrate a highly effective multistage vaccine against both the pre-erythrocytic and sexual stages of Plasmodium falciparum that protects and reduces transmission in a murine model. The vaccine is based on a viral-vectored vaccine platform, comprising a highly-attenuated vaccinia virus strain, LC16m8Δ (m8Δ), a genetically stable variant of a licensed and highly effective Japanese smallpox vaccine LC16m8, and an adeno-associated virus (AAV), a viral vector for human gene therapy. The genes encoding P. falciparum circumsporozoite protein (PfCSP) and the ookinete protein P25 (Pfs25) are expressed as a Pfs25-PfCSP fusion protein, and the heterologous m8Δ-prime/AAV-boost immunization regimen in mice provided both 100% protection against PfCSP-transgenic P. berghei sporozoites and up to 100% transmission blocking efficacy, as determined by a direct membrane feeding assay using parasites from P. falciparum-positive, naturally-infected donors from endemic settings. Remarkably, the persistence of vaccine-induced immune responses were over 7 months and additionally provided complete protection against repeated parasite challenge in a murine model. We propose that application of the m8Δ/AAV malaria multistage vaccine platform has the potential to contribute to the landmark goals of the malaria vaccine technology roadmap, to achieve life-long sterile protection and high-level transmission blocking efficacy.
Keywords: LC16m8Δ; PfCSP; Pfs25; adeno-associated virus (AAV); malaria; plasmodium falciparum; vaccine.
Copyright © 2022 Iyori, Blagborough, Mizuno, Abe, Nagaoka, Hori, Yamagoshi, Da, Gregory, Hasyim, Yamamoto, Sakamoto, Yoshida, Mizukami, Shida and Yoshida.
Conflict of interest statement
The authors have read the journal’s policy and declare the following conflicts of interest: Authors SY, HS, HM and MI are named inventors on filed patents related to viral-vectored malaria vaccines (2022-24221). HS is a named inventor on a filed patent related to LC16m8Δ (WO 2005/054451 A1). Neither of these products has been commercialized. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
A two-dose viral-vectored Plasmodium vivax multistage vaccine confers durable protection and transmission-blockade in a pre-clinical study.Front Immunol. 2024 Apr 30;15:1372584. doi: 10.3389/fimmu.2024.1372584. eCollection 2024. Front Immunol. 2024. PMID: 38745665 Free PMC article.
-
Adeno-associated virus-based malaria booster vaccine following attenuated replication-competent vaccinia virus LC16m8Δ priming.Parasitol Int. 2023 Feb;92:102652. doi: 10.1016/j.parint.2022.102652. Epub 2022 Aug 23. Parasitol Int. 2023. PMID: 36007703
-
Liver-Directed AAV8 Booster Vaccine Expressing Plasmodium falciparum Antigen Following Adenovirus Vaccine Priming Elicits Sterile Protection in a Murine Model.Front Immunol. 2021 Jun 23;12:612910. doi: 10.3389/fimmu.2021.612910. eCollection 2021. Front Immunol. 2021. PMID: 34248928 Free PMC article.
-
Prime-boost vectored malaria vaccines: progress and prospects.Hum Vaccin. 2010 Jan;6(1):78-83. doi: 10.4161/hv.6.1.10116. Epub 2010 Jan 18. Hum Vaccin. 2010. PMID: 20061802 Review.
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
Cited by
-
A two-dose viral-vectored Plasmodium vivax multistage vaccine confers durable protection and transmission-blockade in a pre-clinical study.Front Immunol. 2024 Apr 30;15:1372584. doi: 10.3389/fimmu.2024.1372584. eCollection 2024. Front Immunol. 2024. PMID: 38745665 Free PMC article.
-
A Head-to-Head Comparative Study of the Replication-Competent Vaccinia Virus and AAV1-Based Malaria Vaccine versus RTS,S/AS01 in Murine Models.Vaccines (Basel). 2024 Oct 10;12(10):1155. doi: 10.3390/vaccines12101155. Vaccines (Basel). 2024. PMID: 39460322 Free PMC article.
-
A Novel Ex Vivo Assay to Evaluate Functional Effectiveness of Plasmodium vivax Transmission-Blocking Vaccine Using Pvs25 Transgenic Plasmodium berghei.J Infect Dis. 2024 Jun 14;229(6):1894-1903. doi: 10.1093/infdis/jiae102. J Infect Dis. 2024. PMID: 38408353
References
-
- WHO . World malaria report 2021. Geneva, Switzerland: WHO; (2021).
-
- WHO . More malaria cases and deaths in 2020 linked to Covid-19 disruptions. Geneva, Switzerland: WHO; (2021).
-
- RTS,S Clinical Trials Partnership . Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet (2015) 386(9988):31–45. doi: 10.1016/S0140-6736(15)60721-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

