IL-1Ra gene transfer potentiates BMP2-mediated bone healing by redirecting osteogenesis toward endochondral ossification
- PMID: 36245128
- PMCID: PMC9931547
- DOI: 10.1016/j.ymthe.2022.10.007
IL-1Ra gene transfer potentiates BMP2-mediated bone healing by redirecting osteogenesis toward endochondral ossification
Abstract
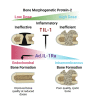
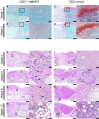
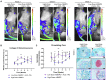
An estimated 100,000 patients each year in the United States suffer severe disability from bone defects that fail to heal, a condition where bone-regenerative therapies could provide substantial clinical benefits. Although recombinant human bone morphogenetic protein-2 (rhBMP2) is an osteogenic growth factor that is clinically approved for this purpose, it is only effective when used at exceedingly high doses that incur substantial costs, induce severe inflammation, produce adverse side effects, and form morphologically abnormal bone. Using a validated rat femoral segmental defect model, we show that bone formed in response to clinically relevant doses of rhBMP2 is accompanied by elevated expression of interleukin-1 (IL-1). Local delivery of cDNA encoding the IL-1 receptor antagonist (IL-1Ra) achieved bridging of segmental, critical size defects in bone with a 90% lower dose of rhBMP2. Unlike use of high-dose rhBMP2, bone formation in the presence of IL-1Ra occurred via the native process of endochondral ossification, resulting in improved quality without sacrificing the mechanical properties of the regenerated bone. Our results demonstrate that local immunomodulation may permit effective use of growth factors at lower doses to recapitulate more precisely the native biology of healing, leading to higher-quality tissue regeneration.
Keywords: bone regeneration; endochondral ossification; gene transfer; interleukin-1 receptor antagonist.
Copyright © 2022 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Similar articles
-
Segmental defect healing in the presence or absence of recombinant human BMP2: Novel insights from a rat model.J Orthop Res. 2023 Sep;41(9):1934-1944. doi: 10.1002/jor.25530. Epub 2023 Feb 27. J Orthop Res. 2023. PMID: 36850029 Free PMC article.
-
Interleukin-1 receptor antagonist enhances the therapeutic efficacy of a low dose of rhBMP-2 in a weight-bearing rat femoral defect model.Acta Biomater. 2022 Sep 1;149:189-197. doi: 10.1016/j.actbio.2022.07.012. Epub 2022 Jul 12. Acta Biomater. 2022. PMID: 35840106
-
Adipose-derived stem cells and BMP2: part 1. BMP2-treated adipose-derived stem cells do not improve repair of segmental femoral defects.Connect Tissue Res. 2011 Apr;52(2):109-18. doi: 10.3109/03008207.2010.484514. Epub 2010 Aug 11. Connect Tissue Res. 2011. PMID: 20701464
-
An activin A/BMP2 chimera, AB204, displays bone-healing properties superior to those of BMP2.J Bone Miner Res. 2014 Sep;29(9):1950-9. doi: 10.1002/jbmr.2238. J Bone Miner Res. 2014. PMID: 24692083 Free PMC article.
-
Exploring calcium-free alternatives in endochondral bone repair tested on In vivo trials - A review.Regen Ther. 2024 Jun 1;26:145-160. doi: 10.1016/j.reth.2024.05.017. eCollection 2024 Jun. Regen Ther. 2024. PMID: 38872977 Free PMC article. Review.
Cited by
-
Impact of PEG sensitization on the efficacy of PEG hydrogel-mediated tissue engineering.Nat Commun. 2024 Apr 18;15(1):3283. doi: 10.1038/s41467-024-46327-3. Nat Commun. 2024. PMID: 38637507 Free PMC article.
-
Cell Reprogramming and Differentiation Utilizing Messenger RNA for Regenerative Medicine.J Dev Biol. 2023 Dec 20;12(1):1. doi: 10.3390/jdb12010001. J Dev Biol. 2023. PMID: 38535481 Free PMC article. Review.
-
Segmental defect healing in the presence or absence of recombinant human BMP2: Novel insights from a rat model.J Orthop Res. 2023 Sep;41(9):1934-1944. doi: 10.1002/jor.25530. Epub 2023 Feb 27. J Orthop Res. 2023. PMID: 36850029 Free PMC article.
-
Research progress of gene therapy combined with tissue engineering to promote bone regeneration.APL Bioeng. 2024 Sep 18;8(3):031502. doi: 10.1063/5.0200551. eCollection 2024 Sep. APL Bioeng. 2024. PMID: 39301183 Free PMC article. Review.
References
-
- U.S. Food & Drug Administration . 2002. Premarket approval (PMA): InFUSE™ bone graft/LT-CAGE™ lumbar taper fusion device. Report No. P000058.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

