Optimized tools and timing of response reassessment after neoadjuvant chemoradiation in rectal cancer
- PMID: 36243807
- PMCID: PMC9569175
- DOI: 10.1007/s00384-022-04268-7
Optimized tools and timing of response reassessment after neoadjuvant chemoradiation in rectal cancer
Abstract
Purpose: Reassessment tools of response to long-course neoadjuvant chemoradiation treatment (nCRT) in patients with locally advanced rectal cancer (LARC) are important in predicting complete response (CR) and thus deciding whether a wait-and-watch strategy can be implemented in these patients. Choosing which routine reassessment tools are optimal and when to use them is still unclear and will be researched in the study.
Methods: Altogether, 250 patients with LARC who received nCRT from 2013 to 2021 and were followed up were retrospectively reviewed. Common reassessment tools of response included digital rectal examination (DRE), clinical examination and symptoms, endoscopy, biopsy, magnetic resonance imaging (MRI), and blood biomarkers.
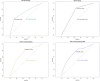
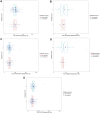
Results: Overall, 27.20% (68/250) patients had a complete response and 72.80% (182/250) did not. The combination of MRI, endoscopy, and biopsy showed the best performance in terms of accuracy of 74% and area under the curve (AUC, 0.714, 95% CI 0.546-0.882). Reassessing through DRE and presence of symptoms failed to improve the efficacy of response reassessment. After 100 days, biopsy as an assessment tool would obtain a substantial rise in accuracy from 51.28 to 100% (p = 0.003).
Conclusion: The combination of MRI, endoscopy, and biopsy is suitable as the reassessment tool of response for applying a wait-and-watch strategy after long-course nCRT in patients with LARC. The accuracy of biopsy as reassessment tools would be improved if they were used over 100 days after nCRT in patients with rectal cancer.
Keywords: Magnetic resonance imaging; Neoadjuvant chemoradiation; Rectal cancer; Response reassessment; Wait and watch strategy.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
["Watch and wait" strategy after neoadjuvant therapy for rectal cancer: status survey of perceptions, attitudes and treatment selection in Chinese surgeons].Zhonghua Wei Chang Wai Ke Za Zhi. 2019 Jun 25;22(6):550-559. doi: 10.3760/cma.j.issn.1671-0274.2019.06.008. Zhonghua Wei Chang Wai Ke Za Zhi. 2019. PMID: 31238634 Chinese.
-
[Application value of colonoscopic assessment in "watch and wait" strategy for mid-lower rectal cancer after neoadjuvant chemoradiotherapy].Zhonghua Wei Chang Wai Ke Za Zhi. 2019 Jul 25;22(7):648-655. doi: 10.3760/cma.j.issn.1671-0274.2019.07.009. Zhonghua Wei Chang Wai Ke Za Zhi. 2019. PMID: 31302963 Chinese.
-
Developing a prediction model based on MRI for pathological complete response after neoadjuvant chemoradiotherapy in locally advanced rectal cancer.Abdom Radiol (NY). 2019 Sep;44(9):2978-2987. doi: 10.1007/s00261-019-02129-6. Abdom Radiol (NY). 2019. PMID: 31327039
-
Surgical treatment following neoadjuvant chemoradiotherapy in locally advanced rectal cancer.Kaohsiung J Med Sci. 2020 Mar;36(3):152-159. doi: 10.1002/kjm2.12161. Epub 2019 Dec 9. Kaohsiung J Med Sci. 2020. PMID: 31814296 Review.
-
A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis.Lancet Gastroenterol Hepatol. 2017 Jul;2(7):501-513. doi: 10.1016/S2468-1253(17)30074-2. Epub 2017 May 4. Lancet Gastroenterol Hepatol. 2017. PMID: 28479372 Review.
References
-
- Jimenez-Fonseca P, Salazar R, Valenti V, Msaouel P, Carmona-Bayonas A. Is short-course radiotherapy and total neoadjuvant therapy the new standard of care in locally advanced rectal cancer? A sensitivity analysis of the RAPIDO clinical trial. Ann Oncol. 2022;33(8):786–793. doi: 10.1016/j.annonc.2022.04.010. - DOI - PubMed
-
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R, German Rectal Cancer Study G Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi: 10.1056/NEJMoa040694. - DOI - PubMed
-
- Jin J, Tang Y, Hu C, Jiang LM, Jiang J, Li N, Liu WY, Chen SL, Li S, Lu NN, Cai Y, Li YH, Zhu Y, Cheng GH, Zhang HY, Wang X, Zhu SY, Wang J, Li GF, Yang JL, Zhang K, Chi Y, Yang L, Zhou HT, Zhou AP, Zou SM, Fang H, Wang SL, Zhang HZ, Wang XS, Wei LC, Wang WL, Liu SX, Gao YH, Li YX. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR) J Clin Oncol. 2022;40(15):1681–1692. doi: 10.1200/JCO.21.01667. - DOI - PMC - PubMed
-
- Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, Allen PK, Lynch PM, Glober G, Wolff R, Rich TA, Skibber J (1999) Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys 44(5):1027–1038. 10.1016/s0360-3016(99)00099-1 - PubMed
MeSH terms
Grants and funding
- D171100006517004/National Key Technology Research and Development Program of the Ministry of Science and Technology of China
- QMS20191103/Young Scholar Program of Beijing Hospitals Authority
- PKU2022LCXQ038/Clinical Medicine Plus X - Young Scholars Project of Peking University
- KC2210/the Science Foundation of Peking University Cancer Hospital
LinkOut - more resources
Full Text Sources

