Current Best Practice in Hepatitis B Management and Understanding Long-term Prospects for Cure
- PMID: 36243037
- PMCID: PMC9772068
- DOI: 10.1053/j.gastro.2022.10.008
Current Best Practice in Hepatitis B Management and Understanding Long-term Prospects for Cure
Abstract
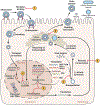
The hepatitis B virus (HBV) is a major cause of cirrhosis and hepatocellular carcinoma worldwide. Despite an effective vaccine, the prevalence of chronic infection remains high. Current therapy is effective at achieving on-treatment, but not off-treatment, viral suppression. Loss of hepatitis B surface antigen, the best surrogate marker of off-treatment viral suppression, is associated with improved clinical outcomes. Unfortunately, this end point is rarely achieved with current therapy because of their lack of effect on covalently closed circular DNA, the template of viral transcription and genome replication. Major advancements in our understanding of HBV virology along with better understanding of immunopathogenesis have led to the development of a multitude of novel therapeutic approaches with the prospect of achieving functional cure (hepatitis B surface antigen loss) and perhaps complete cure (clearance of covalently closed circular DNA and integrated HBV DNA). This review will cover current best practice for managing chronic HBV infection and emerging novel therapies for HBV infection and their prospect for cure.
Keywords: Antiviral; Functional Cure; Screening; Treatment.
Published by Elsevier Inc.
Figures

Similar articles
-
What will it take to cure hepatitis B?Hepatol Commun. 2023 Mar 24;7(4):e0084. doi: 10.1097/HC9.0000000000000084. eCollection 2023 Apr 1. Hepatol Commun. 2023. PMID: 36972391 Free PMC article.
-
Toward a complete cure for chronic hepatitis B: Novel therapeutic targets for hepatitis B virus.Clin Mol Hepatol. 2022 Jan;28(1):17-30. doi: 10.3350/cmh.2021.0093. Epub 2021 Jul 20. Clin Mol Hepatol. 2022. PMID: 34281294 Free PMC article. Review.
-
Mechanism of interferon alpha therapy for chronic hepatitis B and potential approaches to improve its therapeutic efficacy.Antiviral Res. 2024 Jan;221:105782. doi: 10.1016/j.antiviral.2023.105782. Epub 2023 Dec 17. Antiviral Res. 2024. PMID: 38110058 Review.
-
Review article: hepatitis B-current and emerging therapies.Aliment Pharmacol Ther. 2022 Apr;55(7):805-819. doi: 10.1111/apt.16828. Epub 2022 Feb 27. Aliment Pharmacol Ther. 2022. PMID: 35224760 Review.
-
Can we cure hepatitis B virus with novel direct-acting antivirals?Liver Int. 2020 Feb;40 Suppl 1:27-34. doi: 10.1111/liv.14364. Liver Int. 2020. PMID: 32077597 Review.
Cited by
-
Current perspectives of viral hepatitis.World J Gastroenterol. 2024 May 14;30(18):2402-2417. doi: 10.3748/wjg.v30.i18.2402. World J Gastroenterol. 2024. PMID: 38764770 Free PMC article. Review.
-
Lymph node-targeted STING agonist nanovaccine against chronic HBV infection.Cell Mol Life Sci. 2024 Aug 28;81(1):372. doi: 10.1007/s00018-024-05404-y. Cell Mol Life Sci. 2024. PMID: 39196331 Free PMC article.
-
IFN-α induced systemic lupus erythematosus complicated with hemophagocytic lymphohistiocytosis: a case report and literature review.Front Immunol. 2023 Aug 4;14:1223062. doi: 10.3389/fimmu.2023.1223062. eCollection 2023. Front Immunol. 2023. PMID: 37600795 Free PMC article. Review.
-
Overview of Hepatitis B Vaccine Non-Response and Associated B Cell Amnesia: A Scoping Review.Pathogens. 2024 Jul 2;13(7):554. doi: 10.3390/pathogens13070554. Pathogens. 2024. PMID: 39057781 Free PMC article.
-
Symptoms of Liver Disease During Tenofovir Therapy With or Without Peginterferon: Results from the Hepatitis B Research Network Immune Active Trial.Dig Dis Sci. 2023 Dec;68(12):4499-4510. doi: 10.1007/s10620-023-08108-8. Epub 2023 Oct 7. Dig Dis Sci. 2023. PMID: 37804353 Free PMC article.
References
-
- Kocher A, Papac L, Barquera R, Key FM, Spyrou MA, Hübler R, Rohrlach AB, Aron F, Stahl R, Wissgott A et al.: Ten millennia of hepatitis B virus evolution. Science 2021, 374(6564):182–188. - PubMed
-
- Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018, 3(6):383–403. - PubMed
-
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ: Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015, 386(10003):1546–1555. - PubMed
-
- Hepatitis B Fact Sheet [https://www.who.int/news-room/fact-sheets/detail/hepatitis-b]
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

