HIV RNA Screening Reduces Integrase Strand Transfer Inhibitor Resistance Risk in Persons Receiving Long-Acting Cabotegravir for HIV Prevention
- PMID: 36240386
- PMCID: PMC10205624
- DOI: 10.1093/infdis/jiac415
HIV RNA Screening Reduces Integrase Strand Transfer Inhibitor Resistance Risk in Persons Receiving Long-Acting Cabotegravir for HIV Prevention
Abstract
Background: The HPTN 083 trial demonstrated that long-acting cabotegravir (CAB-LA) was superior to tenofovir-disoproxil fumarate/emtricitabine for human immunodeficiency virus (HIV) preexposure prophylaxis (PrEP). Integrase strand transfer inhibitor (INSTI) resistance-associated mutations (RAMs) were detected in some participants with HIV infection. We used a low viral load INSTI genotyping assay to evaluate the timing of emergence of INSTI RAMs and assessed whether HIV screening with a sensitive RNA assay would have detected HIV infection before INSTI resistance emerged.
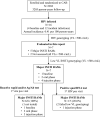
Methods: Single-genome sequencing to detect INSTI RAMs was performed for samples with viral loads <500 copies/mL from 5 participants with previously identified INSTI RAMs and 2 with no prior genotyping results.
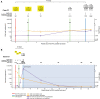
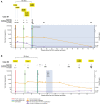
Results: Major INSTI RAMs were detected in all 7 cases. HIV RNA testing identified infection before major INSTI RAMs emerged in 4 cases and before additional major INSTI RAMs accumulated in 1 case. Most INSTI RAMs were detected early when the viral load was low and CAB concentration was high.
Conclusions: When using CAB-LA PrEP, earlier detection of HIV infection with a sensitive RNA assay may allow for earlier treatment initiation with the potential to reduce INSTI resistance risk. Further studies are needed to evaluate the value and feasibility of HIV RNA testing with CAB-LA PrEP.
Keywords: HIV; HPTN; INSTI; PrEP; cabotegravir; injectable; integrase; prevention; resistance.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. R. J. L. has served on scientific advisory boards for Merck and has received honoraria from Roche and Janssen. A. R. and M. S. C. are employees of ViiV Healthcare. J. W. M. is a consultant to Gilead Sciences, owns shares of Abound Bio, Inc, and share options in Infectious Disease Connect. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


Similar articles
-
Efficacy and safety of long-acting cabotegravir compared with daily oral tenofovir disoproxil fumarate plus emtricitabine to prevent HIV infection in cisgender men and transgender women who have sex with men 1 year after study unblinding: a secondary analysis of the phase 2b and 3 HPTN 083 randomised controlled trial.Lancet HIV. 2023 Dec;10(12):e767-e778. doi: 10.1016/S2352-3018(23)00261-8. Epub 2023 Nov 9. Lancet HIV. 2023. PMID: 37952550 Free PMC article. Clinical Trial.
-
Genotypic correlates of resistance to the HIV-1 strand transfer integrase inhibitor cabotegravir.Antiviral Res. 2022 Dec;208:105427. doi: 10.1016/j.antiviral.2022.105427. Epub 2022 Sep 30. Antiviral Res. 2022. PMID: 36191692 Free PMC article.
-
Long-Acting Injectable Cabotegravir for HIV Prevention: What Do We Know and Need to Know about the Risks and Consequences of Cabotegravir Resistance?Curr HIV/AIDS Rep. 2022 Oct;19(5):384-393. doi: 10.1007/s11904-022-00616-y. Epub 2022 Sep 16. Curr HIV/AIDS Rep. 2022. PMID: 36112336 Free PMC article. Review.
-
Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women.N Engl J Med. 2021 Aug 12;385(7):595-608. doi: 10.1056/NEJMoa2101016. N Engl J Med. 2021. PMID: 34379922 Free PMC article. Clinical Trial.
-
A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection.Retrovirology. 2022 Oct 22;19(1):22. doi: 10.1186/s12977-022-00608-1. Retrovirology. 2022. PMID: 36273165 Free PMC article. Review.
Cited by
-
First Case of HIV Seroconversion With Integrase Resistance Mutations on Long-Acting Cabotegravir for Prevention in Routine Care.Open Forum Infect Dis. 2024 Aug 26;11(9):ofae468. doi: 10.1093/ofid/ofae468. eCollection 2024 Sep. Open Forum Infect Dis. 2024. PMID: 39229286 Free PMC article.
-
Evaluation of Xpert point-of-care assays for detection of HIV infection in persons using long-acting cabotegravir for pre-exposure prophylaxis.Microbiol Spectr. 2024 Jul 9;12(8):e0030724. doi: 10.1128/spectrum.00307-24. Online ahead of print. Microbiol Spectr. 2024. PMID: 38980027 Free PMC article.
-
HIV Pre-Exposure Prophylaxis: New and Upcoming Drugs to Address the HIV Epidemic.Drugs. 2023 Dec;83(18):1677-1698. doi: 10.1007/s40265-023-01963-9. Epub 2023 Dec 11. Drugs. 2023. PMID: 38079092 Review.
-
Efficacy and safety of long-acting cabotegravir compared with daily oral tenofovir disoproxil fumarate plus emtricitabine to prevent HIV infection in cisgender men and transgender women who have sex with men 1 year after study unblinding: a secondary analysis of the phase 2b and 3 HPTN 083 randomised controlled trial.Lancet HIV. 2023 Dec;10(12):e767-e778. doi: 10.1016/S2352-3018(23)00261-8. Epub 2023 Nov 9. Lancet HIV. 2023. PMID: 37952550 Free PMC article. Clinical Trial.
-
Need assessment for HIV drug resistance testing and landscape of current and future technologies in low- and middle-income countries.PLOS Glob Public Health. 2023 Oct 18;3(10):e0001948. doi: 10.1371/journal.pgph.0001948. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 37851634 Free PMC article. Review.
References
-
- ViiV Healthcare Limited . Apretude (package insert). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf. Accessed 18 February 2022.
-
- World Health Organization . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach, 2021. https://www.who.int/publications/i/item/9789240031593. Accessed 18 February 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

