Cellular shape reinforces niche to stem cell signaling in the small intestine
- PMID: 36240269
- PMCID: PMC9565803
- DOI: 10.1126/sciadv.abm1847
Cellular shape reinforces niche to stem cell signaling in the small intestine
Abstract
Niche-derived factors regulate tissue stem cells, but apart from the mechanosensory pathways, the effect of niche geometry is not well understood. We used organoids and bioengineered tissue culture platforms to demonstrate that the conical shape of Lgr5+ small intestinal stem cells (ISCs) facilitate their self-renewal and function. Inhibition of non-muscle myosin II (NM II)-driven apical constriction altered ISC shape and reduced niche curvature and stem cell capacity. Niche curvature is decreased in aged mice, suggesting that suboptimal interactions between old ISCs and their niche develop with age. We show that activation of NM IIC or physical restriction to young topology improves in vitro regeneration by old epithelium. We propose that the increase in lateral surface area of ISCs induced by apical constriction promotes interactions between neighboring cells, and the curved topology of the intestinal niche has evolved to maximize signaling between ISCs and neighboring cells.
Figures

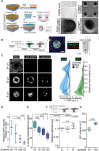

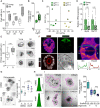
Similar articles
-
Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration.Gastroenterology. 2020 Sep;159(3):956-968.e8. doi: 10.1053/j.gastro.2020.05.067. Epub 2020 May 30. Gastroenterology. 2020. PMID: 32485177
-
Krüppel-like Factor 5 Regulates Stemness, Lineage Specification, and Regeneration of Intestinal Epithelial Stem Cells.Cell Mol Gastroenterol Hepatol. 2020;9(4):587-609. doi: 10.1016/j.jcmgh.2019.11.009. Epub 2019 Nov 25. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31778829 Free PMC article.
-
Paneth cell-derived iNOS is required to maintain homeostasis in the intestinal stem cell niche.J Transl Med. 2023 Nov 25;21(1):852. doi: 10.1186/s12967-023-04744-w. J Transl Med. 2023. PMID: 38007452 Free PMC article.
-
The cellular niche for intestinal stem cells: a team effort.Cell Regen. 2021 Jan 1;10(1):1. doi: 10.1186/s13619-020-00061-5. Cell Regen. 2021. PMID: 33385259 Free PMC article. Review.
-
Plasticity of Intestinal Epithelium: Stem Cell Niches and Regulatory Signals.Int J Mol Sci. 2020 Dec 31;22(1):357. doi: 10.3390/ijms22010357. Int J Mol Sci. 2020. PMID: 33396437 Free PMC article. Review.
Cited by
-
Gut aging: A wane from the normal to repercussion and gerotherapeutic strategies.Heliyon. 2024 Sep 12;10(19):e37883. doi: 10.1016/j.heliyon.2024.e37883. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39381110 Free PMC article. Review.
-
An actomyosin network organizes niche morphology and responds to feedback from recruited stem cells.bioRxiv [Preprint]. 2024 Aug 7:2023.09.08.556877. doi: 10.1101/2023.09.08.556877. bioRxiv. 2024. Update in: Curr Biol. 2024 Sep 9;34(17):3917-3930.e6. doi: 10.1016/j.cub.2024.07.041. PMID: 38746236 Free PMC article. Updated. Preprint.
-
Advancing Synthetic Hydrogels through Nature-Inspired Materials Chemistry.Adv Mater. 2024 Oct;36(42):e2404235. doi: 10.1002/adma.202404235. Epub 2024 Jul 1. Adv Mater. 2024. PMID: 38896849 Review.
-
Toward Corneal Limbus In Vitro Model: Regulation of hPSC-LSC Phenotype by Matrix Stiffness and Topography During Cell Differentiation Process.Adv Healthc Mater. 2023 Nov;12(29):e2301396. doi: 10.1002/adhm.202301396. Epub 2023 Jul 21. Adv Healthc Mater. 2023. PMID: 37449943 Free PMC article.
-
The Drosophila hematopoietic niche assembles through collective cell migration controlled by neighbor tissues and Slit-Robo signaling.bioRxiv [Preprint]. 2024 Oct 15:2024.06.21.600069. doi: 10.1101/2024.06.21.600069. bioRxiv. 2024. Update in: Elife. 2025 Jan 03;13:RP100455. doi: 10.7554/eLife.100455. PMID: 38979182 Free PMC article. Updated. Preprint.
References
-
- Chacon-Martinez C. A., Koester J., Wickstrom S. A., Signaling in the stem cell niche: Regulating cell fate, function and plasticity. Development 145, dev165399 (2018). - PubMed
-
- Guignard L., Fiúza U.-M., Leggio B., Laussu J., Faure E., Michelin G., Biasuz K., Hufnagel L., Malandain G., Godin C., Lemaire P., Contact area-dependent cell communication and the morphological invariance of ascidian embryogenesis. Science 369, eaar5663 (2020). - PubMed
-
- Barker N., Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 15, 19–33 (2014). - PubMed
-
- Pentinmikko N., Iqbal S., Mana M., Andersson S., Cognetta A. B. III, Suciu R. M., Roper J., Luopajärvi K., Markelin E., Gopalakrishnan S., Smolander O.-P., Naranjo S., Saarinen T., Juuti A., Pietiläinen K., Auvinen P., Ristimäki A., Gupta N., Tammela T., Jacks T., Sabatini D. M., Cravatt B. F., Yilmaz Ö. H., Katajisto P., Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 571, 398–402 (2019). - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

