Advances in IgA glycosylation and its correlation with diseases
- PMID: 36238099
- PMCID: PMC9552352
- DOI: 10.3389/fchem.2022.974854
Advances in IgA glycosylation and its correlation with diseases
Abstract
Immunoglobulin A (IgA) is the most abundant immunoglobulin synthesized in the human body. It has the highest concentration in the mucosa and is second only to IgG in serum. IgA plays an important role in mucosal immunity, and is the predominant antibody used to protect the mucosal surface from pathogens invasion and to maintain the homeostasis of intestinal flora. Moreover, The binding IgA to the FcαRI (Fc alpha Receptor I) in soluble or aggregated form can mediate anti- or pro- inflammatory responses, respectively. IgA is also known as one of the most heavily glycosylated antibodies among human immunoglobulins. The glycosylation of IgA has been shown to have a significant effect on its immune function. Variation in the glycoform of IgA is often the main characteration of autoimmune diseases such as IgA nephropathy (IgAN), IgA vasculitis (IgAV), systemic lupus erythematosus (SLE), and rheumatoid arthritis (RA). However, compared with the confirmed glycosylation function of IgG, the pathogenic mechanism of IgA glycosylation involved in related diseases is still unclear. This paper mainly summarizes the recent reports on IgA's glycan structure, its function, its relationship with the occurrence and development of diseases, and the potential application of glycoengineered IgA in clinical antibody therapeutics, in order to provide a potential reference for future research in this field.
Keywords: IgA; antibody; glycosylation; immunoglobulin; therapy.
Copyright © 2022 Ding, Chen, Cheng, Zhang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
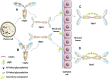
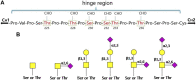
 : GalNAc;
: GalNAc;  : Gal;
: Gal;  : Sia) (Novak et al., 2000; Novak et al., 2018; Hansen et al., 2021).
: Sia) (Novak et al., 2000; Novak et al., 2018; Hansen et al., 2021).Similar articles
-
The era of the immunoglobulin A Fc receptor FcαRI; its function and potential as target in disease.Immunol Rev. 2015 Nov;268(1):123-38. doi: 10.1111/imr.12337. Immunol Rev. 2015. PMID: 26497517 Review.
-
Interaction of antigens and antibodies at mucosal surfaces.Annu Rev Microbiol. 1997;51:311-40. doi: 10.1146/annurev.micro.51.1.311. Annu Rev Microbiol. 1997. PMID: 9343353 Review.
-
Anti-agalactosyl IgG antibodies and isotype profiles of rheumatoid factors in Sjögren's syndrome and rheumatoid arthritis.Clin Exp Rheumatol. 1998 Nov-Dec;16(6):709-15. Clin Exp Rheumatol. 1998. PMID: 9844764
-
Immunoglobulin A Glycosylation and Its Role in Disease.Exp Suppl. 2021;112:433-477. doi: 10.1007/978-3-030-76912-3_14. Exp Suppl. 2021. PMID: 34687019
-
Distinct Fcα receptor N-glycans modulate the binding affinity to immunoglobulin A (IgA) antibodies.J Biol Chem. 2019 Sep 20;294(38):13995-14008. doi: 10.1074/jbc.RA119.009954. Epub 2019 Jul 30. J Biol Chem. 2019. PMID: 31362986 Free PMC article.
Cited by
-
Identification of new drugs to counteract anti-spike IgG-induced hyperinflammation in severe COVID-19.Life Sci Alliance. 2023 Sep 12;6(11):e202302106. doi: 10.26508/lsa.202302106. Print 2023 Nov. Life Sci Alliance. 2023. PMID: 37699657 Free PMC article.
-
The IgA of hares (Lepus sp.) and rabbit confirms that the leporids IgA explosion is old and reveals a new case of trans-species polymorphism.Front Immunol. 2023 Aug 3;14:1192460. doi: 10.3389/fimmu.2023.1192460. eCollection 2023. Front Immunol. 2023. PMID: 37600766 Free PMC article.
-
The Exploitation of the Glycosylation Pattern in Asthma: How We Alter Ancestral Pathways to Develop New Treatments.Biomolecules. 2024 Apr 24;14(5):513. doi: 10.3390/biom14050513. Biomolecules. 2024. PMID: 38785919 Free PMC article. Review.
-
Antibody Aggregation: A Problem Within the Biopharmaceutical Industry and Its Role in AL Amyloidosis Disease.Protein J. 2024 Nov 11. doi: 10.1007/s10930-024-10237-6. Online ahead of print. Protein J. 2024. PMID: 39527351 Review.
-
N-glycosylation of immunoglobulin A in children and adults with type 1 diabetes mellitus.Heliyon. 2024 May 3;10(9):e30529. doi: 10.1016/j.heliyon.2024.e30529. eCollection 2024 May 15. Heliyon. 2024. PMID: 38765169 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

