Adipocyte commitment of 3T3-L1 cells is required to support human adenovirus 36 productive replication concurrent with altered lipid and glucose metabolism
- PMID: 36237435
- PMCID: PMC9553024
- DOI: 10.3389/fcimb.2022.1016200
Adipocyte commitment of 3T3-L1 cells is required to support human adenovirus 36 productive replication concurrent with altered lipid and glucose metabolism
Abstract
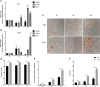
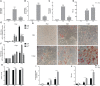
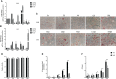
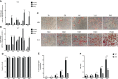
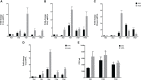
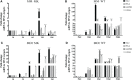
Human adenovirus 36 (HAdV-D36) can cause obesity in animal models, induces an adipogenic effect and increased adipocyte differentiation in cell culture. HAdV-D36 infection alters gene expression and the metabolism of the infected cells resulting in increased glucose internalization and triglyceride accumulation. Although HAdV-D36 prevalence correlates with obesity in humans, whether human preadipocytes may be targeted in vivo has not been determined and metabolic reprogramming of preadipocytes has not been explored in the context of the viral replication cycle. HAdV-D36 infection of the mouse fibroblasts, 3T3-L1 cells, which can differentiate into adipocytes, promotes proliferation and differentiation, but replication of the virus in these cells is abortive as indicated by short-lived transient expression of viral mRNA and a progressive loss of viral DNA. Therefore, we have evaluated whether a productive viral replication cycle can be established in the 3T3-L1 preadipocyte model under conditions that drive the cell differentiation process. For this purpose, viral mRNA levels and viral DNA replication were measured by RT-qPCR and qPCR, respectively, and viral progeny production was determined by plaque assay. The lipogenic effect of infection was evaluated with Oil Red O (ORO) staining, and expression of genes that control lipid and glucose metabolism was measured by RT-qPCR. In the context of a viral productive cycle, HAdV-D36 modulated the expression of the adipogenic genes, C/EBPα, C/EBPβ and PPARγ, as well as intracellular lipid accumulation, and the infection was accompanied by altered expression of glucolytic genes. The results show that only adipocyte-committed 3T3-L1 cells are permissive for the expression of early and late viral mRNAs, as well as viral DNA replication and progeny production, supporting productive HAdV-D36 viral replication, indicating that a greater effect on adipogenesis occurs in adipocytes that support productive viral replication.
Keywords: adipocyte commitment; human adenovirus 36; lipid and glucose metabolism; obesity; permissive replication.
Copyright © 2022 Márquez, Ballesteros, Dobner and González.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Viral mRNA expression but not DNA replication is required for lipogenic effect of human adenovirus Ad-36 in preadipocytes.Int J Obes (Lond). 2007 Jan;31(1):78-86. doi: 10.1038/sj.ijo.0803358. Epub 2006 May 2. Int J Obes (Lond). 2007. PMID: 16652125
-
Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells.Lipids Health Dis. 2017 Sep 25;16(1):181. doi: 10.1186/s12944-017-0574-7. Lipids Health Dis. 2017. PMID: 28946872 Free PMC article.
-
Adipogenic human adenovirus-36 reduces leptin expression and secretion and increases glucose uptake by fat cells.Int J Obes (Lond). 2007 Jan;31(1):87-96. doi: 10.1038/sj.ijo.0803366. Epub 2006 May 16. Int J Obes (Lond). 2007. PMID: 16703005
-
Metabolic Reprogramming of the Host Cell by Human Adenovirus Infection.Viruses. 2019 Feb 8;11(2):141. doi: 10.3390/v11020141. Viruses. 2019. PMID: 30744016 Free PMC article. Review.
-
E4orf1: The triple agent of adenovirus - Unraveling its roles in oncogenesis, infectious obesity and immune responses in virus replication and vector therapy.Tumour Virus Res. 2024 Jun;17:200277. doi: 10.1016/j.tvr.2024.200277. Epub 2024 Feb 28. Tumour Virus Res. 2024. PMID: 38428735 Free PMC article. Review.
Cited by
-
Pharmacomicrobiomics and Drug-Infection Interactions: The Impact of Commensal, Symbiotic and Pathogenic Microorganisms on a Host Response to Drug Therapy.Int J Mol Sci. 2023 Dec 4;24(23):17100. doi: 10.3390/ijms242317100. Int J Mol Sci. 2023. PMID: 38069427 Free PMC article. Review.
References
-
- Afruza R., Akheruzzaman M., Dhurandhar N. V., Hegde V. (2020). E4orf1, an adeno-viral protein, attenuates renal lipid accumulation in high fat fed mice: A novel approach to reduce a key risk factor for chronic kidney disease. Heliyon 6 (10), e05261. doi: 10.1016/j.heliyon.2020.e05261 - DOI - PMC - PubMed
-
- Berk A. J. (2013). Adenoviridae chap. 55 in B. N., Knipe D. M., Howley P. M., "Adenoviridae Chap. 55" in Howley, Fields Virology. 6th Edition. Eds B.N, Knipe D.M., P.M (Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; ).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

