Role of myeloid-derived suppressor cells in the formation of pre-metastatic niche
- PMID: 36237333
- PMCID: PMC9552826
- DOI: 10.3389/fonc.2022.975261
Role of myeloid-derived suppressor cells in the formation of pre-metastatic niche
Abstract
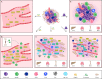
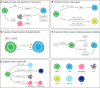
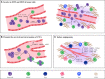
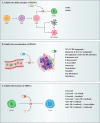
Metastasis is a complex process, which depends on the interaction between tumor cells and host organs. Driven by the primary tumor, the host organ will establish an environment suitable for the growth of tumor cells before their arrival, which is called the pre-metastasis niche. The formation of pre-metastasis niche requires the participation of a variety of cells, in which myeloid-derived suppressor cells play a very important role. They reach the host organ before the tumor cells, and promote the establishment of the pre-metastasis niche by influencing immunosuppression, vascular leakage, extracellular matrix remodeling, angiogenesis and so on. In this article, we introduced the formation of the pre-metastasis niche and discussed the important role of myeloid-derived suppressor cells. In addition, this paper also emphasized the targeting of myeloid-derived suppressor cells as a therapeutic strategy to inhibit the formation of pre-metastasis niche, which provided a research idea for curbing tumor metastasis.
Keywords: circulating tumor cells; immunosuppression; myeloid-derived suppressor cells; pre-metastatic niche; targeted therapy.
Copyright © 2022 Ya, Ren, Qin, He and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Pre-metastatic niche: formation, characteristics and therapeutic implication.Signal Transduct Target Ther. 2024 Sep 25;9(1):236. doi: 10.1038/s41392-024-01937-7. Signal Transduct Target Ther. 2024. PMID: 39317708 Free PMC article. Review.
-
Sponge-like nano-system suppresses tumor recurrence and metastasis by restraining myeloid-derived suppressor cells-mediated immunosuppression and formation of pre-metastatic niche.Acta Biomater. 2023 Mar 1;158:708-724. doi: 10.1016/j.actbio.2023.01.009. Epub 2023 Jan 11. Acta Biomater. 2023. PMID: 36638937
-
MDSCs: The Key Players in the Formation of Pre-Metastatic Niche.Front Biosci (Landmark Ed). 2023 Mar 20;28(3):58. doi: 10.31083/j.fbl2803058. Front Biosci (Landmark Ed). 2023. PMID: 37005751 Review.
-
Pre-metastatic Niche Formation by Neutrophils in Different Organs.Adv Exp Med Biol. 2021;1329:93-108. doi: 10.1007/978-3-030-73119-9_5. Adv Exp Med Biol. 2021. PMID: 34664235
-
Myeloid regulatory cells in tumor spreading and metastasis.Immunobiology. 2015 Feb;220(2):236-42. doi: 10.1016/j.imbio.2014.07.017. Epub 2014 Jul 23. Immunobiology. 2015. PMID: 25178934 Review.
Cited by
-
Xiaoliu Pingyi Pecipe Inhibits Lung Pre-Metastatic Niche Formation and Prevents Myeloid-Derived Suppressor Cells Recruitment.Integr Cancer Ther. 2023 Jan-Dec;22:15347354231187000. doi: 10.1177/15347354231187000. Integr Cancer Ther. 2023. PMID: 37431869 Free PMC article.
-
Identification of novel diagnostic biomarkers associated with liver metastasis in colon adenocarcinoma by machine learning.Discov Oncol. 2024 Oct 10;15(1):542. doi: 10.1007/s12672-024-01398-y. Discov Oncol. 2024. PMID: 39390264 Free PMC article.
-
Pre-metastatic niche: formation, characteristics and therapeutic implication.Signal Transduct Target Ther. 2024 Sep 25;9(1):236. doi: 10.1038/s41392-024-01937-7. Signal Transduct Target Ther. 2024. PMID: 39317708 Free PMC article. Review.
-
Emerging viral infections in immunocompromised patients: A great challenge to better define the role of immune response.Front Immunol. 2023 Mar 9;14:1147871. doi: 10.3389/fimmu.2023.1147871. eCollection 2023. Front Immunol. 2023. PMID: 36969202 Free PMC article. Review.
-
Immunometabolic reprogramming, another cancer hallmark.Front Immunol. 2023 May 19;14:1125874. doi: 10.3389/fimmu.2023.1125874. eCollection 2023. Front Immunol. 2023. PMID: 37275901 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

