Semisynthetic Derivatives of Pentacyclic Triterpenes Bearing Heterocyclic Moieties with Therapeutic Potential
- PMID: 36235089
- PMCID: PMC9572482
- DOI: 10.3390/molecules27196552
Semisynthetic Derivatives of Pentacyclic Triterpenes Bearing Heterocyclic Moieties with Therapeutic Potential
Abstract
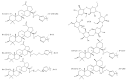
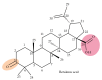
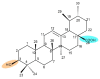
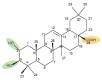
Medicinal plants have been used by humans since ancient times for the treatment of various diseases and currently represent the main source of a variety of phytocompounds, such as triterpenes. Pentacyclic triterpenes have been subjected to numerous studies that have revealed various biological activities, such as anticancer, antidiabetic, anti-inflammatory, antimicrobial, and hepatoprotective effects, which can be employed in therapy. However, due to their high lipophilicity, which is considered to exert a significant influence on their bioavailability, their current use is limited. A frequent approach employed to overcome this obstacle is the chemical derivatization of the core structure with different types of moieties including heterocycles, which are considered key elements in medicinal chemistry. The present review aims to summarize the literature published in the last 10 years regarding the derivatives of pentacyclic triterpenes bearing heterocyclic moieties and focuses on the biologically active derivatives as well as their structure-activity relationships. Predominantly, the targeted positions for the derivatization of the triterpene skeleton are C-3 (hydroxyl/oxo group), C-28 (hydroxyl/carboxyl group), and C-30 (allylic group) or the extension of the main scaffold by fusing various heterocycles with the A-ring of the phytocompound. In addition, numerous derivatives also contain linker moieties that connect the triterpenic scaffold with heterocycles; one such linker, the triazole moiety, stands out as a key pharmacophore for its biological effect. All these studies support the hypothesis that triterpenoid conjugates with heterocyclic moieties may represent promising candidates for future clinical trials.
Keywords: betulin; betulinic acid; betulonic acid; bioconjugates; corosolic acid; heterocycles; lupeol; maslinic acid; oleanolic acid; pentacyclic triterpenes; structure–activity relationship; ursolic acid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Pentacyclic Triterpenoids with Nitrogen-Containing Heterocyclic Moiety, Privileged Hybrids in Anticancer Drug Discovery.Molecules. 2021 Apr 21;26(9):2401. doi: 10.3390/molecules26092401. Molecules. 2021. PMID: 33918996 Free PMC article. Review.
-
Novel Triterpenic Acid-Benzotriazole Esters Act as Pro-Apoptotic Antimelanoma Agents.Int J Mol Sci. 2022 Sep 1;23(17):9992. doi: 10.3390/ijms23179992. Int J Mol Sci. 2022. PMID: 36077389 Free PMC article.
-
Optimization of the derivatization protocol of pentacyclic triterpenes prior to their gas chromatography-mass spectrometry analysis in plant extracts.Talanta. 2016 Jan 15;147:35-43. doi: 10.1016/j.talanta.2015.09.026. Epub 2015 Sep 13. Talanta. 2016. PMID: 26592573
-
Pentacyclic triterpenes as α-glucosidase and α-amylase inhibitors: Structure-activity relationships and the synergism with acarbose.Bioorg Med Chem Lett. 2017 Nov 15;27(22):5065-5070. doi: 10.1016/j.bmcl.2017.09.027. Epub 2017 Sep 14. Bioorg Med Chem Lett. 2017. PMID: 28964635
-
Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy.Planta Med. 2009 Dec;75(15):1549-60. doi: 10.1055/s-0029-1186102. Planta Med. 2009. PMID: 19742422 Review.
Cited by
-
Synthesis of Rhodamine-Conjugated Lupane Type Triterpenes of Enhanced Cytotoxicity.Molecules. 2024 May 16;29(10):2346. doi: 10.3390/molecules29102346. Molecules. 2024. PMID: 38792206 Free PMC article.
-
Synthesis and Anticancer Activity of A-Ring-Modified Derivatives of Dihydrobetulin.Int J Mol Sci. 2023 Jun 7;24(12):9863. doi: 10.3390/ijms24129863. Int J Mol Sci. 2023. PMID: 37373011 Free PMC article.
-
Enhancing bioavailability of natural extracts for nutritional applications through dry powder inhalers (DPI) spray drying: technological advancements and future directions.Front Nutr. 2023 Jul 5;10:1190912. doi: 10.3389/fnut.2023.1190912. eCollection 2023. Front Nutr. 2023. PMID: 37476406 Free PMC article. Review.
-
Cytotoxic Potential of Betulinic Acid Fatty Esters and Their Liposomal Formulations: Targeting Breast, Colon, and Lung Cancer Cell Lines.Molecules. 2024 Jul 19;29(14):3399. doi: 10.3390/molecules29143399. Molecules. 2024. PMID: 39064977 Free PMC article.
-
Isolation and identification of novel selective antitumor constituents, sidrin and sidroside, from Zizyphus spina-christi.Saudi Pharm J. 2023 Jun;31(6):1019-1028. doi: 10.1016/j.jsps.2023.04.029. Epub 2023 May 6. Saudi Pharm J. 2023. PMID: 37234346 Free PMC article.
References
-
- Saxena M., Saxena J., Nema R., Singh D., Gupta A. Phytochemistry of Medicinal Plants. J. Pharmacogn. Phytochem. 2013;1:168–182.
-
- Soica C., Voicu M., Ghiulai R., Dehelean C., Racoviceanu R., Trandafirescu C., Rosca O.-J., Nistor G., Mioc M., Mioc A. Natural Compounds in Sex Hormone-Dependent Cancers: The Role of Triterpenes as Therapeutic Agents. Front. Endocrinol. 2021;11:612396. doi: 10.3389/fendo.2020.612396. - DOI - PMC - PubMed
-
- Ghiulai R., Roşca O.J., Antal D.S., Mioc M., Mioc A., Racoviceanu R., Macaşoi I., Olariu T., Dehelean C., Creţu O.M., et al. Tetracyclic and pentacyclic triterpenes with high therapeutic efficiency in wound healing approaches. Molecules. 2020;25:5557. doi: 10.3390/molecules25235557. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

