Carvedilol Selectively Stimulates βArrestin2-Dependent SERCA2a Activity in Cardiomyocytes to Augment Contractility
- PMID: 36232617
- PMCID: PMC9570329
- DOI: 10.3390/ijms231911315
Carvedilol Selectively Stimulates βArrestin2-Dependent SERCA2a Activity in Cardiomyocytes to Augment Contractility
Abstract
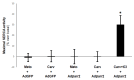
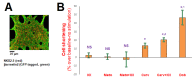
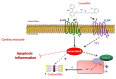
Heart failure (HF) carries the highest mortality in the western world and β-blockers [β-adrenergic receptor (AR) antagonists] are part of the cornerstone pharmacotherapy for post-myocardial infarction (MI) chronic HF. Cardiac β1AR-activated βarrestin2, a G protein-coupled receptor (GPCR) adapter protein, promotes Sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2a SUMO (small ubiquitin-like modifier)-ylation and activity, thereby directly increasing cardiac contractility. Given that certain β-blockers, such as carvedilol and metoprolol, can activate βarrestins and/or SERCA2a in the heart, we investigated the effects of these two agents on cardiac βarrestin2-dependent SERCA2a SUMOylation and activity. We found that carvedilol, but not metoprolol, acutely induces βarrestin2 interaction with SERCA2a in H9c2 cardiomyocytes and in neonatal rat ventricular myocytes (NRVMs), resulting in enhanced SERCA2a SUMOylation. However, this translates into enhanced SERCA2a activity only in the presence of the β2AR-selective inverse agonist ICI 118,551 (ICI), indicating an opposing effect of carvedilol-occupied β2AR subtype on carvedilol-occupied β1AR-stimulated, βarrestin2-dependent SERCA2a activation. In addition, the amplitude of fractional shortening of NRVMs, transfected to overexpress βarrestin2, is acutely enhanced by carvedilol, again in the presence of ICI only. In contrast, metoprolol was without effect on NRVMs' shortening amplitude irrespective of ICI co-treatment. Importantly, the pro-contractile effect of carvedilol was also observed in human induced pluripotent stem cell (hIPSC)-derived cardiac myocytes (CMs) overexpressing βarrestin2, and, in fact, it was present even without concomitant ICI treatment of human CMs. Metoprolol with or without concomitant ICI did not affect contractility of human CMs, either. In conclusion, carvedilol, but not metoprolol, stimulates βarrestin2-mediated SERCA2a SUMOylation and activity through the β1AR in cardiac myocytes, translating into direct positive inotropy. However, this unique βarrestin2-dependent pro-contractile effect of carvedilol may be opposed or masked by carvedilol-bound β2AR subtype signaling.
Keywords: G protein-coupled receptor; SERACA2a; SUMOylation; cardiomyocyte; contractility; signal transduction; β-adrenergic receptor; βarrestin2.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
β-Arrestin2 Improves Post-Myocardial Infarction Heart Failure via Sarco(endo)plasmic Reticulum Ca2+-ATPase-Dependent Positive Inotropy in Cardiomyocytes.Hypertension. 2017 Nov;70(5):972-981. doi: 10.1161/HYPERTENSIONAHA.117.09817. Epub 2017 Sep 5. Hypertension. 2017. PMID: 28874462
-
Carvedilol induces biased β1 adrenergic receptor-nitric oxide synthase 3-cyclic guanylyl monophosphate signalling to promote cardiac contractility.Cardiovasc Res. 2021 Aug 29;117(10):2237-2251. doi: 10.1093/cvr/cvaa266. Cardiovasc Res. 2021. PMID: 32956449 Free PMC article.
-
Effect of beta-blockers on cardiac function and calcium handling protein in postinfarction heart failure rats.Chest. 2005 Sep;128(3):1812-21. doi: 10.1378/chest.128.3.1812. Chest. 2005. PMID: 16162791
-
[Modification of sarco-endoplasmic reticulum Ca(2 +) -ATPase in the failing cardiomyocyte].Clin Calcium. 2013 Apr;23(4):535-42. Clin Calcium. 2013. PMID: 23545743 Review. Japanese.
-
SERCA2a: a key protein in the Ca2+ cycle of the heart failure.Heart Fail Rev. 2020 May;25(3):523-535. doi: 10.1007/s10741-019-09873-3. Heart Fail Rev. 2020. PMID: 31701344 Review.
Cited by
-
Investigating the effects of beta-blockers on circadian heart rhythm using heart rate variability in ischemic heart disease with preserved ejection fraction.Sci Rep. 2023 Apr 10;13(1):5828. doi: 10.1038/s41598-023-32963-0. Sci Rep. 2023. PMID: 37037871 Free PMC article.
-
Cellulase with Bacillus velezensis improves physicochemical characteristics, microbiota and metabolites of corn germ meal during two-stage co-fermentation.World J Microbiol Biotechnol. 2024 Jan 3;40(2):59. doi: 10.1007/s11274-023-03831-w. World J Microbiol Biotechnol. 2024. PMID: 38170296
-
Role of G-Protein-Coupled Receptors in Cardiovascular Diseases.Int J Mol Sci. 2023 Apr 24;24(9):7760. doi: 10.3390/ijms24097760. Int J Mol Sci. 2023. PMID: 37175466 Free PMC article.
-
Efficacy of the New Inotropic Agent Istaroxime in Acute Heart Failure.J Clin Med. 2022 Dec 18;11(24):7503. doi: 10.3390/jcm11247503. J Clin Med. 2022. PMID: 36556120 Free PMC article. Review.
-
Insights Into the Role of Angiotensin-II AT1 Receptor-Dependent β-Arrestin Signaling in Cardiovascular Disease.Hypertension. 2024 Jan;81(1):6-16. doi: 10.1161/HYPERTENSIONAHA.123.19419. Epub 2023 Jul 14. Hypertension. 2024. PMID: 37449411 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

