Fcα Receptor-1-Activated Monocytes Promote B Lymphocyte Migration and IgA Isotype Switching
- PMID: 36232432
- PMCID: PMC9569671
- DOI: 10.3390/ijms231911132
Fcα Receptor-1-Activated Monocytes Promote B Lymphocyte Migration and IgA Isotype Switching
Abstract
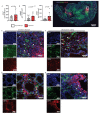
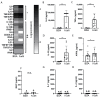
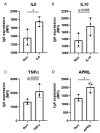
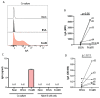
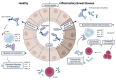
Patients with inflammatory bowel disease (IBD) produce enhanced immunoglobulin A (IgA) against the microbiota compared to healthy individuals, which has been correlated with disease severity. Since IgA complexes can potently activate myeloid cells via the IgA receptor FcαRI (CD89), excessive IgA production may contribute to IBD pathology. However, the cellular mechanisms that contribute to dysregulated IgA production in IBD are poorly understood. Here, we demonstrate that intestinal FcαRI-expressing myeloid cells (i.e., monocytes and neutrophils) are in close contact with B lymphocytes in the lamina propria of IBD patients. Furthermore, stimulation of FcαRI-on monocytes triggered production of cytokines and chemokines that regulate B-cell differentiation and migration, including interleukin-6 (IL6), interleukin-10 (IL10), tumour necrosis factor-α (TNFα), a proliferation-inducing ligand (APRIL), and chemokine ligand-20 (CCL20). In vitro, these cytokines promoted IgA isotype switching in human B cells. Moreover, when naïve B lymphocytes were cultured in vitro in the presence of FcαRI-stimulated monocytes, enhanced IgA isotype switching was observed compared to B cells that were cultured with non-stimulated monocytes. Taken together, FcαRI-activated monocytes produced a cocktail of cytokines, as well as chemokines, that stimulated IgA switching in B cells, and close contact between B cells and myeloid cells was observed in the colons of IBD patients. As such, we hypothesize that, in IBD, IgA complexes activate myeloid cells, which in turn can result in excessive IgA production, likely contributing to disease pathology. Interrupting this loop may, therefore, represent a novel therapeutic strategy.
Keywords: CCL20; CD89; FcαRI; IBD; IgA; immunoglobulin A; inflammatory bowel disease; monocytes; myeloid cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Differential expression and function of IgA receptors (CD89 and CD71) during maturation of dendritic cells.J Leukoc Biol. 2004 Dec;76(6):1134-41. doi: 10.1189/jlb.0204101. Epub 2004 Sep 15. J Leukoc Biol. 2004. PMID: 15371488
-
IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4.J Immunol. 2008 Aug 1;181(3):1767-79. doi: 10.4049/jimmunol.181.3.1767. J Immunol. 2008. PMID: 18641314
-
IgA and FcαRI: Pathological Roles and Therapeutic Opportunities.Front Immunol. 2019 Mar 22;10:553. doi: 10.3389/fimmu.2019.00553. eCollection 2019. Front Immunol. 2019. PMID: 30984170 Free PMC article. Review.
-
Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells.Clin Exp Immunol. 2005 Jun;140(3):478-90. doi: 10.1111/j.1365-2249.2005.02779.x. Clin Exp Immunol. 2005. PMID: 15932509 Free PMC article.
-
IgA Fc receptors.Annu Rev Immunol. 2003;21:177-204. doi: 10.1146/annurev.immunol.21.120601.141011. Epub 2001 Dec 19. Annu Rev Immunol. 2003. PMID: 12524384 Review.
Cited by
-
Type I/II Immune Balance Contributes to the Protective Effect of AIF-1 on Hepatic Immunopathology Induced by Schistosoma japonicum in a Transgenic Mouse Model.Inflammation. 2024 Oct;47(5):1806-1819. doi: 10.1007/s10753-024-02010-9. Epub 2024 Mar 30. Inflammation. 2024. PMID: 38554240
-
Research advancements and perspectives of inflammatory bowel disease: A comprehensive review.Sci Prog. 2024 Apr-Jun;107(2):368504241253709. doi: 10.1177/00368504241253709. Sci Prog. 2024. PMID: 38778725 Free PMC article. Review.
-
The Role of IgA in the Manifestation and Prevention of Allergic Immune Responses.Curr Allergy Asthma Rep. 2023 Oct;23(10):589-600. doi: 10.1007/s11882-023-01105-x. Epub 2023 Aug 23. Curr Allergy Asthma Rep. 2023. PMID: 37610671 Free PMC article. Review.
References
-
- He B., Xu W., Santini P.A., Polydorides A.D., Chiu A., Estrella J., Shan M., Chadburn A., Villanacci V., Plebani A., et al. Intestinal Bacteria Trigger T Cell-Independent Immunoglobulin A2 Class Switching by Inducing Epithelial-Cell Secretion of the Cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

