Senescence of Tumor Cells in Anticancer Therapy-Beneficial and Detrimental Effects
- PMID: 36232388
- PMCID: PMC9570404
- DOI: 10.3390/ijms231911082
Senescence of Tumor Cells in Anticancer Therapy-Beneficial and Detrimental Effects
Abstract
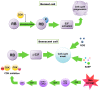
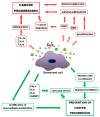
Cellular senescence process results in stable cell cycle arrest, which prevents cell proliferation. It can be induced by a variety of stimuli including metabolic stress, DNA damage, telomeres shortening, and oncogenes activation. Senescence is generally considered as a process of tumor suppression, both by preventing cancer cells proliferation and inhibiting cancer progression. It can also be a key effector mechanism for many types of anticancer therapies such as chemotherapy and radiotherapy, both directly and through bioactive molecules released by senescent cells that can stimulate an immune response. Senescence is characterized by a senescence-associated secretory phenotype (SASP) that can have both beneficial and detrimental impact on cancer progression. Despite the negatives, attempts are still being made to use senescence to fight cancer, especially when it comes to senolytics. There is a possibility that a combination of prosenescence therapy-which targets tumor cells and causes their senescence-with senotherapy-which targets senescent cells, can be promising in cancer treatment. This review provides information on cellular senescence, its connection with carcinogenesis and therapeutic possibilities linked to this process.
Keywords: SASP; cancer; prosenescence therapy; senescence; senolysis; senolytics; senostatics; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Senescent cells in cancer therapy: why and how to remove them.Cancer Lett. 2021 Nov 1;520:68-79. doi: 10.1016/j.canlet.2021.07.002. Epub 2021 Jul 5. Cancer Lett. 2021. PMID: 34237406 Review.
-
The role of cellular senescence and SASP in tumour microenvironment.FEBS J. 2023 Mar;290(5):1348-1361. doi: 10.1111/febs.16381. Epub 2022 Feb 20. FEBS J. 2023. PMID: 35106956 Review.
-
Biological functions of therapy-induced senescence in cancer.Semin Cancer Biol. 2022 Jun;81:5-13. doi: 10.1016/j.semcancer.2021.03.021. Epub 2021 Mar 26. Semin Cancer Biol. 2022. PMID: 33775830
-
The Potential of Senescence as a Target for Developing Anticancer Therapy.Int J Mol Sci. 2023 Feb 8;24(4):3436. doi: 10.3390/ijms24043436. Int J Mol Sci. 2023. PMID: 36834846 Free PMC article. Review.
-
Senescent cells and SASP in cancer microenvironment: New approaches in cancer therapy.Adv Protein Chem Struct Biol. 2023;133:115-158. doi: 10.1016/bs.apcsb.2022.10.002. Epub 2022 Nov 25. Adv Protein Chem Struct Biol. 2023. PMID: 36707199 Review.
Cited by
-
Hepatitis B virus X protein and TGF-β: partners in the carcinogenic journey of hepatocellular carcinoma.Front Oncol. 2024 Jun 19;14:1407434. doi: 10.3389/fonc.2024.1407434. eCollection 2024. Front Oncol. 2024. PMID: 38962270 Free PMC article. Review.
-
Platinum iodido drugs show potential anti-tumor activity, affecting cancer cell metabolism and inducing ROS and senescence in gastrointestinal cancer cells.Commun Biol. 2024 Mar 22;7(1):353. doi: 10.1038/s42003-024-06052-5. Commun Biol. 2024. PMID: 38519773 Free PMC article.
-
Therapy-Induced Cellular Senescence: Potentiating Tumor Elimination or Driving Cancer Resistance and Recurrence?Cells. 2024 Jul 30;13(15):1281. doi: 10.3390/cells13151281. Cells. 2024. PMID: 39120312 Free PMC article. Review.
-
Senescent cell-derived vaccines: a new concept towards an immune response against cancer and aging?Aging (Albany NY). 2024 Jun 26;16(12):10657-10665. doi: 10.18632/aging.205975. Epub 2024 Jun 26. Aging (Albany NY). 2024. PMID: 38942604 Free PMC article. Review.
-
Fibroblast growth factor receptor signaling in estrogen receptor-positive breast cancer: mechanisms and role in endocrine resistance.Front Oncol. 2024 Jul 8;14:1406951. doi: 10.3389/fonc.2024.1406951. eCollection 2024. Front Oncol. 2024. PMID: 39040443 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

