Change in effectiveness of sotrovimab for preventing hospitalization and mortality for at-risk COVID-19 outpatients during an Omicron BA.1 and BA.1.1-predominant phase
- PMID: 36229005
- PMCID: PMC9549713
- DOI: 10.1016/j.ijid.2022.10.002
Change in effectiveness of sotrovimab for preventing hospitalization and mortality for at-risk COVID-19 outpatients during an Omicron BA.1 and BA.1.1-predominant phase
Abstract
Objectives: Sotrovimab effectively prevented progression to severe disease and mortality following infection with pre-Omicron SARS-CoV-2 variants. We sought to determine whether sotrovimab is similarly effective against SARS-CoV-2 Omicron variant infection.
Methods: Observational cohort study of non-hospitalized adult patients with SARS-CoV-2 infection from December 26, 2021, to March 10, 2022, using electronic health records from a statewide health system. We propensity-matched patients not receiving authorized treatment for each patient treated with sotrovimab. The primary outcome was 28-day hospitalization; secondary outcomes included mortality. We also propensity-matched sotrovimab-treated patients from the Omicron and Delta phases. Logistic regression was used to determine sotrovimab effectiveness during Omicron and between variant phases.
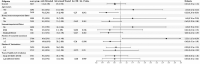
Results: Of 30,247 SARS-CoV-2 Omicron variant infected outpatients, we matched 1542 receiving sotrovimab to 3663 not receiving treatment. Sotrovimab treatment was not associated with reduced odds of 28-day hospitalization (2.5% vs 3.2%; adjusted odds ratio [OR] 0.82, 95% CI 0.55, 1.19) or mortality (0.1% vs 0.2%; adjusted OR 0.62, 95% CI 0.07, 2.78). Between phases, the observed treatment OR was higher during Omicron than during Delta (OR 0.85 vs 0.39, respectively; interaction P-value = 0.053).
Conclusion: Real-world evidence demonstrated that sotrovimab was not associated with reduced 28-day hospitalization or mortality among COVID-19 outpatients during the Omicron BA.1 phase.
Keywords: COVID-19; Monoclonal antibody; Real-world evidence.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors do not have a commercial or other association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding).
Figures
Similar articles
-
Real-World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients.J Infect Dis. 2022 Dec 13;226(12):2129-2136. doi: 10.1093/infdis/jiac206. J Infect Dis. 2022. PMID: 35576581 Free PMC article.
-
Real World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients.medRxiv [Preprint]. 2022 Apr 5:2022.04.03.22273360. doi: 10.1101/2022.04.03.22273360. medRxiv. 2022. Update in: J Infect Dis. 2022 Dec 13;226(12):2129-2136. doi: 10.1093/infdis/jiac206. PMID: 35411339 Free PMC article. Updated. Preprint.
-
Real-World Effectiveness of Sotrovimab for the Early Treatment of COVID-19 During SARS-CoV-2 Delta and Omicron Waves in the USA.Infect Dis Ther. 2023 Feb;12(2):607-621. doi: 10.1007/s40121-022-00755-0. Epub 2023 Jan 11. Infect Dis Ther. 2023. PMID: 36629998 Free PMC article.
-
Real-world effectiveness of sotrovimab for the treatment of SARS-CoV-2 infection during Omicron BA.2 subvariant predominance: a systematic literature review.Infection. 2024 Feb;52(1):1-17. doi: 10.1007/s15010-023-02098-5. Epub 2023 Sep 30. Infection. 2024. PMID: 37776474 Free PMC article. Review.
-
Sotrovimab use in Japanese inpatients with COVID-19: post-infusion adverse events.BMC Infect Dis. 2022 Dec 3;22(1):902. doi: 10.1186/s12879-022-07889-z. BMC Infect Dis. 2022. PMID: 36461006 Free PMC article. Review.
Cited by
-
Efficacy and safety of sotrovimab in patients with COVID-19: A rapid review and meta-analysis.Rev Med Virol. 2022 Nov;32(6):e2402. doi: 10.1002/rmv.2402. Epub 2022 Oct 12. Rev Med Virol. 2022. PMID: 36226323 Free PMC article. Review.
-
Monitoring of Sotrovimab-Levels as Pre-Exposure Prophylaxis in Kidney Transplant Recipients Not Responding to SARS-CoV-2 Vaccines.Viruses. 2023 Jul 26;15(8):1624. doi: 10.3390/v15081624. Viruses. 2023. PMID: 37631967 Free PMC article.
-
Real-world evaluation of bebtelovimab effectiveness during the period of COVID-19 Omicron variants, including BA.4/BA.5.Int J Infect Dis. 2023 Jul;132:34-39. doi: 10.1016/j.ijid.2023.04.396. Epub 2023 Apr 16. Int J Infect Dis. 2023. PMID: 37072054 Free PMC article.
-
Sotrovimab: A Review of Its Efficacy against SARS-CoV-2 Variants.Viruses. 2024 Jan 31;16(2):217. doi: 10.3390/v16020217. Viruses. 2024. PMID: 38399991 Free PMC article. Review.
-
Outpatient Treatment of Confirmed COVID-19: Living, Rapid Practice Points From the American College of Physicians (Version 2).Ann Intern Med. 2023 Oct;176(10):1396-1404. doi: 10.7326/M23-1636. Epub 2023 Sep 19. Ann Intern Med. 2023. PMID: 37722112 Free PMC article.
References
-
- Aggarwal NR, Beaty LE, Bennett TD, Carlson NE, Davis CB, Kwan BM, Mayer DA, Ong TC, Russell S, Steele J, Wogu AF, Wynia MK, Zane RD, Ginde AA. Real world evidence of the neutralizing monoclonal antibody sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients. J Infect Dis. 2022;226(12):2129–2136. doi: 10.1093/infdis/jiac206. - DOI - PMC - PubMed
-
PMID: 35576581
-
- Angus DC. Optimizing the trade-off between learning and doing in a pandemic. JAMA. 2020;323:1895–1896. - PubMed
-
- Bennett TD, Moffitt RA, Hajagos JG, Amor B, Anand A, Bissell MM, Bradwell KR, Bremer C, Byrd JB, Denham A, DeWitt PE, Gabriel D, Garibaldi BT, Girvin AT, Guinney J, Hill EL, Hong SS, Jimenez H, Kavuluru R, Kostka K, Lehmann HP, Levitt E, Mallipattu SK, Manna A, McMurry JA, Morris M, Muschelli J, Neumann AJ, Palchuk MB, Pfaff ER, Qian Z, Qureshi N, Russell S, Spratt H, Walden A, Williams AE, Wooldridge JT, Yoo YJ, Zhang XT, Zhu RL, Austin CP, Saltz JH, Gersing KR, Haendel MA, Chute CG, National COVID Cohort Collaborative (N3C) Consortium Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US national COVID cohort collaborative. JAMA Netw Open. 2021;4 - PMC - PubMed
-
- Colorado Department of Public Health and Environment . 2021. COVID-19 Treatments.https://covid19.colorado.gov/for-coloradans/covid-19-treatments#collapse... accessed 10 June YYYY.
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

