The novel visual cycle inhibitor (±)-RPE65-61 protects retinal photoreceptors from light-induced degeneration
- PMID: 36227868
- PMCID: PMC9560169
- DOI: 10.1371/journal.pone.0269437
The novel visual cycle inhibitor (±)-RPE65-61 protects retinal photoreceptors from light-induced degeneration
Abstract
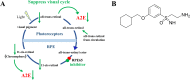
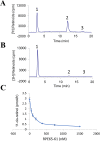
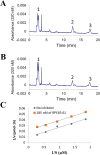
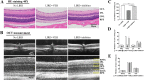
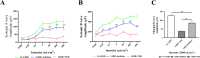
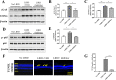
The visual cycle refers to a series of biochemical reactions of retinoids in ocular tissues and supports the vision in vertebrates. The visual cycle regenerates visual pigments chromophore, 11-cis-retinal, and eliminates its toxic byproducts from the retina, supporting visual function and retinal neuron survival. Unfortunately, during the visual cycle, when 11-cis-retinal is being regenerated in the retina, toxic byproducts, such as all-trans-retinal and bis-retinoid is N-retinylidene-N-retinylethanolamine (A2E), are produced, which are proposed to contribute to the pathogenesis of the dry form of age-related macular degeneration (AMD). The primary biochemical defect in Stargardt disease (STGD1) is the accelerated synthesis of cytotoxic lipofuscin bisretinoids, such as A2E, in the retinal pigment epithelium (RPE) due to mutations in the ABCA4 gene. To prevent all-trans-retinal-and bisretinoid-mediated retinal degeneration, slowing down the retinoid flow by modulating the visual cycle with a small molecule has been proposed as a therapeutic strategy. The present study describes RPE65-61, a novel, non-retinoid compound, as an inhibitor of RPE65 (a key enzyme in the visual cycle), intended to modulate the excessive activity of the visual cycle to protect the retina from harm degenerative diseases. Our data demonstrated that (±)-RPE65-61 selectively inhibited retinoid isomerase activity of RPE65, with an IC50 of 80 nM. Furthermore, (±)-RPE65-61 inhibited RPE65 via an uncompetitive mechanism. Systemic administration of (±)-RPE65-61 in mice resulted in slower chromophore regeneration after light bleach, confirming in vivo target engagement and visual cycle modulation. Concomitant protection of the mouse retina from high-intensity light damage was also observed. Furthermore, RPE65-61 down-regulated the cyclic GMP-AMP synthase stimulator of interferon genes (cGAS-STING) pathway, decreased the inflammatory factor, and attenuated retinal apoptosis caused by light-induced retinal damage (LIRD), which led to the preservation of the retinal function. Taken together, (±)-RPE65-61 is a potent visual cycle modulator that may provide a neuroprotective therapeutic benefit for patients with STGD and AMD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Visual Cycle Modulation as an Approach toward Preservation of Retinal Integrity.PLoS One. 2015 May 13;10(5):e0124940. doi: 10.1371/journal.pone.0124940. eCollection 2015. PLoS One. 2015. PMID: 25970164 Free PMC article.
-
A novel RPE65 inhibitor CU239 suppresses visual cycle and prevents retinal degeneration.Biochim Biophys Acta Mol Basis Dis. 2018 Jul;1864(7):2420-2429. doi: 10.1016/j.bbadis.2018.04.014. Epub 2018 Apr 21. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29684583 Free PMC article.
-
Inhibition of the visual cycle by A2E through direct interaction with RPE65 and implications in Stargardt disease.Proc Natl Acad Sci U S A. 2010 Oct 12;107(41):17551-6. doi: 10.1073/pnas.1008769107. Epub 2010 Sep 27. Proc Natl Acad Sci U S A. 2010. PMID: 20876139 Free PMC article.
-
Macular Pigment Carotenoids and Bisretinoid A2E.Adv Exp Med Biol. 2023;1415:15-20. doi: 10.1007/978-3-031-27681-1_3. Adv Exp Med Biol. 2023. PMID: 37440008 Review.
-
Vitamin A and Vision.Subcell Biochem. 2016;81:231-259. doi: 10.1007/978-94-024-0945-1_9. Subcell Biochem. 2016. PMID: 27830507 Review.
Cited by
-
The Role of Retinal Pigment Epithelial Cells in Age-Related Macular Degeneration: Phagocytosis and Autophagy.Biomolecules. 2023 May 29;13(6):901. doi: 10.3390/biom13060901. Biomolecules. 2023. PMID: 37371481 Free PMC article. Review.
-
Tuning the Metabolic Stability of Visual Cycle Modulators through Modification of an RPE65 Recognition Motif.J Med Chem. 2023 Jun 22;66(12):8140-8158. doi: 10.1021/acs.jmedchem.3c00461. Epub 2023 Jun 6. J Med Chem. 2023. PMID: 37279401 Free PMC article.
References
-
- Golczak M, Maeda A, Bereta G, Maeda T, Kiser PD, Hunzelmann S, et al.. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J Biol Chem. 2008;283(15):9543–54. Epub 2008/01/16. M708982200 [pii]. 10.1074/jbc.M708982200. doi: 10.1074/jbc.M708982200 ; PubMed Central PMCID: PMC2441898. - DOI - PMC - PubMed
-
- Rattner A, Smallwood PM, Nathans J. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000;275(15):11034–43. doi: 10.1074/jbc.275.15.11034 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

