N-Glycosylation on Asn50 of SND1 Is Required for Glioma U87 Cell Proliferation and Metastasis
- PMID: 36213325
- PMCID: PMC9537018
- DOI: 10.1155/2022/5239006
N-Glycosylation on Asn50 of SND1 Is Required for Glioma U87 Cell Proliferation and Metastasis
Abstract
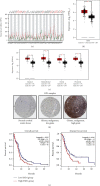
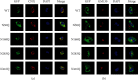
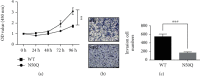
Staphylococcal nuclease domain-containing protein 1 (SND1) is an evolutionarily conserved multidomain protein, which has gained attention recently due to its positive regulation in several cancer progression and metastatic spread. However, the specific contribution of SND1 glycosylation in glioma remains uncertain. In the current study, we confirmed that SND1 was highly expressed in human glioma. Using site-directed mutagenesis, we created four predicted N-glycosylation site mutants for SND1 and provided the first evidence that SND1 undergoes N-glycosylation on its Asn50, Asn168, Asn283, and Asn416 residues in human glioma U87 cells. In addition, we found that removing the N-glycans on the Asn50 site destabilized SND1 and led to its endoplasmic reticulum-associated degradation. Furthermore, destabilized SND1 inhibits the glioma cell proliferation and metastasis. Collectively, our results reveal that N-glycosylation at Asn50 is essential for SND1 folding and trafficking, thus essential for the glioma process, providing new insights for SND1 as a potential disease biomarker for glioma.
Copyright © 2022 Ying Zhou et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
The novel chromatin architectural regulator SND1 promotes glioma proliferation and invasion and predicts the prognosis of patients.Neuro Oncol. 2019 Jun 10;21(6):742-754. doi: 10.1093/neuonc/noz038. Neuro Oncol. 2019. PMID: 30753603 Free PMC article.
-
Suppression of miR-184 in malignant gliomas upregulates SND1 and promotes tumor aggressiveness.Neuro Oncol. 2015 Mar;17(3):419-29. doi: 10.1093/neuonc/nou220. Epub 2014 Sep 12. Neuro Oncol. 2015. PMID: 25216670 Free PMC article.
-
Mechanism for Bioactive Nanomaterial circ0024831 Regulation of Staphylococcal Nuclease Domain Containing 1 via RNA Methylation Recognition in Osteosarcoma.J Biomed Nanotechnol. 2022 Feb 1;18(2):453-462. doi: 10.1166/jbn.2022.3256. J Biomed Nanotechnol. 2022. PMID: 35484754
-
Insights Into SND1 Oncogene Promoter Regulation.Front Oncol. 2018 Dec 11;8:606. doi: 10.3389/fonc.2018.00606. eCollection 2018. Front Oncol. 2018. PMID: 30619748 Free PMC article. Review.
-
Molecular and cellular insights into the role of SND1 in lipid metabolism.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 May;1865(5):158589. doi: 10.1016/j.bbalip.2019.158589. Epub 2020 Jan 21. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31978555 Review.
Cited by
-
Screening and identifying of biomarkers in early colorectal cancer and adenoma based on genome-wide methylation profiles.World J Surg Oncol. 2023 Oct 2;21(1):312. doi: 10.1186/s12957-023-03189-1. World J Surg Oncol. 2023. PMID: 37779184 Free PMC article.
References
-
- Stupp R., Hegi M. E., Mason W. P., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology . 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

