Development and validation of chest CT-based imaging biomarkers for early stage COVID-19 screening
- PMID: 36211676
- PMCID: PMC9533142
- DOI: 10.3389/fpubh.2022.1004117
Development and validation of chest CT-based imaging biomarkers for early stage COVID-19 screening
Abstract
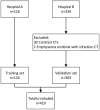
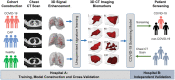
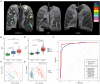
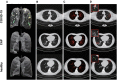
Coronavirus Disease 2019 (COVID-19) is currently a global pandemic, and early screening is one of the key factors for COVID-19 control and treatment. Here, we developed and validated chest CT-based imaging biomarkers for COVID-19 patient screening from two independent hospitals with 419 patients. We identified the vasculature-like signals from CT images and found that, compared to healthy and community acquired pneumonia (CAP) patients, COVID-19 patients display a significantly higher abundance of these signals. Furthermore, unsupervised feature learning led to the discovery of clinical-relevant imaging biomarkers from the vasculature-like signals for accurate and sensitive COVID-19 screening that have been double-blindly validated in an independent hospital (sensitivity: 0.941, specificity: 0.920, AUC: 0.971, accuracy 0.931, F1 score: 0.929). Our findings could open a new avenue to assist screening of COVID-19 patients.
Keywords: Coronavirus Disease 2019 (COVID-19); artificial intelligence; biomedical imaging application; chest CT image; imaging biomarker; multicentric retrospective study.
Copyright © 2022 Liu, Yang, Xiong, Mao, Jin, Li, Zhou and Chang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
From community-acquired pneumonia to COVID-19: a deep learning-based method for quantitative analysis of COVID-19 on thick-section CT scans.Eur Radiol. 2020 Dec;30(12):6828-6837. doi: 10.1007/s00330-020-07042-x. Epub 2020 Jul 18. Eur Radiol. 2020. PMID: 32683550 Free PMC article.
-
3D CT-Inclusive Deep-Learning Model to Predict Mortality, ICU Admittance, and Intubation in COVID-19 Patients.J Digit Imaging. 2023 Apr;36(2):603-616. doi: 10.1007/s10278-022-00734-4. Epub 2022 Nov 30. J Digit Imaging. 2023. PMID: 36450922 Free PMC article.
-
A novel deep learning-based method for COVID-19 pneumonia detection from CT images.BMC Med Inform Decis Mak. 2022 Nov 2;22(1):284. doi: 10.1186/s12911-022-02022-1. BMC Med Inform Decis Mak. 2022. PMID: 36324135 Free PMC article.
-
Computed tomography-based COVID-19 triage through a deep neural network using mask-weighted global average pooling.Front Cell Infect Microbiol. 2023 Mar 3;13:1116285. doi: 10.3389/fcimb.2023.1116285. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36936770 Free PMC article.
-
An original deep learning model using limited data for COVID-19 discrimination: A multicenter study.Med Phys. 2022 Jun;49(6):3874-3885. doi: 10.1002/mp.15549. Epub 2022 Apr 18. Med Phys. 2022. PMID: 35305027 Free PMC article.
Cited by
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2021 Mar 16;3(3):CD013639. doi: 10.1002/14651858.CD013639.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 16;5:CD013639. doi: 10.1002/14651858.CD013639.pub5. PMID: 33724443 Free PMC article. Updated.
-
A Learning-Based Model to Evaluate Hospitalization Priority in COVID-19 Pandemics.Patterns (N Y). 2020 Sep 11;1(6):100092. doi: 10.1016/j.patter.2020.100092. Epub 2020 Aug 3. Patterns (N Y). 2020. PMID: 32838344 Free PMC article.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2022 May 16;5(5):CD013639. doi: 10.1002/14651858.CD013639.pub5. Cochrane Database Syst Rev. 2022. PMID: 35575286 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

