Development and use of a high-throughput screen to identify novel modulators of the corticotropin releasing factor binding protein
- PMID: 36210051
- PMCID: PMC9762412
- DOI: 10.1016/j.slasd.2022.09.005
Development and use of a high-throughput screen to identify novel modulators of the corticotropin releasing factor binding protein
Abstract
Background: Stress responses are believed to involve corticotropin releasing factor (CRF), its two cognate receptors (CRF1 and CRF2), and the CRF-binding protein (CRFBP). Whereas decades of research has focused on CRF1, the role of CRF2 in the central nervous system (CNS) has not been thoroughly investigated. We have previously reported that CRF2, interacting with a C terminal fragment of CRFBP, CRFBP(10kD), may have a role in the modulation of neuronal activity. However, the mechanism by which CRF interacts with CRFBP(10kD) and CRF2 has not been fully elucidated due to the lack of useful chemical tools to probe CRFBP.
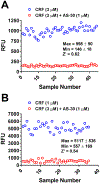
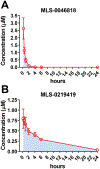
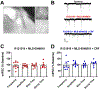
Methods: We miniaturized a cell-based assay, where CRFBP(10kD) is fused as a chimera with CRF2, and performed a high-throughput screen (HTS) of 350,000 small molecules to find negative allosteric modulators (NAMs) of the CRFBP(10kD)-CRF2 complex. Hits were confirmed by evaluating activity toward parental HEK293 cells, toward CRF2 in the absence of CRFBP(10kD), and toward CRF1 in vitro. Hits were further characterized in ex vivo electrophysiology assays that target: 1) the CRF1+ neurons in the central nucleus of the amygdala (CeA) of CRF1:GFP mice that express GFP under the CRF1 promoter, and 2) the CRF-induced potentiation of N-methyl-D-aspartic acid receptor (NMDAR)-mediated synaptic transmission in dopamine neurons in the ventral tegmental area (VTA).
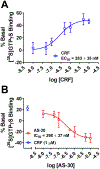
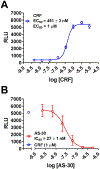
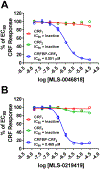
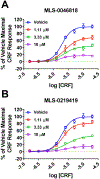
Results: We found that CRFBP(10kD) potentiates CRF-intracellular Ca2+ release specifically via CRF2, indicating that CRFBP may possess excitatory roles in addition to the inhibitory role established by the N-terminal fragment of CRFBP, CRFBP(27kD). We identified novel small molecule CRFBP-CRF2 NAMs that do not alter the CRF1-mediated effects of exogenous CRF but blunt CRF-induced potentiation of NMDAR-mediated synaptic transmission in dopamine neurons in the VTA, an effect mediated by CRF2 and CRFBP.
Conclusion: These results provide the first evidence of specific roles for CRF2 and CRFBP(10kD) in the modulation of neuronal activity and suggest that CRFBP(10kD)-CRF2 NAMs can be further developed for the treatment of stress-related disorders including alcohol and substance use disorders.
Keywords: AUD; CRF(2); CRFBP; HTS; NAM; Stress.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interests LHS is currently an employee of Fate Therapeutics, San Diego, CA, United States. SV and BSH are currently employees of Alchem Laboratories Corp, Alachua, FL, United States. ES is currently an employee of Chugai Pharmaceutical Co. Ltd., Tokyo, Japan. MPH is currently an employee of Boundless Bio, La Jolla, CA, United States. MM is an employee of Kite Pharmaceuticals, CA, United States. AB is currently an employee of GIA (Global Institutes on Addiction), Miami, FL. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Corticotropin Releasing Factor Binding Protein and CRF2 Receptors in the Ventral Tegmental Area: Modulation of Ethanol Binge Drinking in C57BL/6J Mice.Alcohol Clin Exp Res. 2015 Sep;39(9):1609-18. doi: 10.1111/acer.12825. Epub 2015 Aug 6. Alcohol Clin Exp Res. 2015. PMID: 26247973 Free PMC article.
-
Sex differences in CRF1, CRF, and CRFBP expression in C57BL/6J mouse brain across the lifespan and in response to acute stress.J Neurochem. 2021 Aug;158(4):943-959. doi: 10.1111/jnc.15157. Epub 2020 Sep 17. J Neurochem. 2021. PMID: 32813270 Free PMC article.
-
Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior.J Neurosci. 2004 Jul 21;24(29):6545-52. doi: 10.1523/JNEUROSCI.5760-03.2004. J Neurosci. 2004. PMID: 15269266 Free PMC article.
-
Recent advances with the CRF1 receptor: design of small molecule inhibitors, receptor subtypes and clinical indications.Curr Pharm Des. 1999 May;5(5):289-315. Curr Pharm Des. 1999. PMID: 10213797 Review.
-
Corticotropin-releasing factor receptor type 1 and type 2 interaction in irritable bowel syndrome.J Gastroenterol. 2015 Aug;50(8):819-30. doi: 10.1007/s00535-015-1086-8. Epub 2015 May 12. J Gastroenterol. 2015. PMID: 25962711 Review.
Cited by
-
Neuroscience targets and human studies: future translational efforts in the stress system.Neuropsychopharmacology. 2023 Apr;48(5):711-712. doi: 10.1038/s41386-023-01539-x. Epub 2023 Feb 1. Neuropsychopharmacology. 2023. PMID: 36725866 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
- R33 DA029966/DA/NIDA NIH HHS/United States
- K99 MH123673/MH/NIMH NIH HHS/United States
- K01 AA023867/AA/NIAAA NIH HHS/United States
- P30 CA030199/CA/NCI NIH HHS/United States
- P20 GM130414/GM/NIGMS NIH HHS/United States
- T32 AA007456/AA/NIAAA NIH HHS/United States
- FI2 GM128622/GM/NIGMS NIH HHS/United States
- HHSN271201300017C/MH/NIMH NIH HHS/United States
- R21 AA027614/AA/NIAAA NIH HHS/United States
- R01 AA021491/AA/NIAAA NIH HHS/United States
- R01 AA027760/AA/NIAAA NIH HHS/United States
- U54 HG005033/HG/NHGRI NIH HHS/United States
- R01 AA026589/AA/NIAAA NIH HHS/United States
- R01 AA029841/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

