Poly-L-ornithine blocks the inhibitory effects of fibronectin on oligodendrocyte differentiation and promotes myelin repair
- PMID: 36204851
- PMCID: PMC9700116
- DOI: 10.4103/1673-5374.353493
Poly-L-ornithine blocks the inhibitory effects of fibronectin on oligodendrocyte differentiation and promotes myelin repair
Abstract
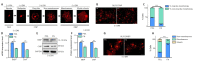
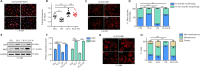
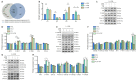
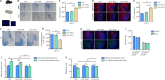
The extracellular matrix surrounding oligodendrocytes plays an important role during myelination and remyelination in the brain. In many cases, the microenvironment surrounding demyelination lesions contains inhibitory molecules, which lead to repair failure. Accordingly, blocking the activity of these inhibitory factors in the extracellular matrix should lead to more successful remyelination. In the central nervous system, oligodendrocytes form the myelin sheath. We performed primary cell culture and found that a natural increase in fibronectin promoted the proliferation of oligodendrocyte progenitors during the initial stage of remyelination while inhibiting oligodendrocyte differentiation. Poly-L-ornithine blocked these inhibitory effects without compromising fibronectin's pro-proliferation function. Experiments showed that poly-L-ornithine activated the Erk1/2 signaling pathway that is necessary in the early stages of differentiation, as well as PI3K signaling pathways that are needed in the mid-late stages. When poly-L-ornithine was tested in a lysolecithin-induced animal model of focal demyelination, it enhanced myelin regeneration and promoted motor function recovery. These findings suggest that poly-L-ornithine has the potential to be a treatment option for clinical myelin sheath injury.
Keywords: Erk1/2; PI3K; differentiation; extracellular matrix; fibronectin; lysolecithin-induced demyelination; oligodendrocyte; poly-L-ornithine; proliferation; remyelination.
Conflict of interest statement
None
Figures


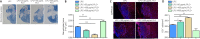
Similar articles
-
Olig2-Targeted G-Protein-Coupled Receptor Gpr17 Regulates Oligodendrocyte Survival in Response to Lysolecithin-Induced Demyelination.J Neurosci. 2016 Oct 12;36(41):10560-10573. doi: 10.1523/JNEUROSCI.0898-16.2016. J Neurosci. 2016. PMID: 27733608 Free PMC article.
-
The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review.
-
Matrix metalloproteinases shape the oligodendrocyte (niche) during development and upon demyelination.Neurosci Lett. 2020 Jun 11;729:134980. doi: 10.1016/j.neulet.2020.134980. Epub 2020 Apr 19. Neurosci Lett. 2020. PMID: 32315713 Review.
-
Loss of Tuberous Sclerosis Complex1 in Adult Oligodendrocyte Progenitor Cells Enhances Axon Remyelination and Increases Myelin Thickness after a Focal Demyelination.J Neurosci. 2017 Aug 2;37(31):7534-7546. doi: 10.1523/JNEUROSCI.3454-16.2017. Epub 2017 Jul 10. J Neurosci. 2017. PMID: 28694334 Free PMC article.
-
The Extracellular Matrix Proteins Tenascin-C and Tenascin-R Retard Oligodendrocyte Precursor Maturation and Myelin Regeneration in a Cuprizone-Induced Long-Term Demyelination Animal Model.Cells. 2022 May 28;11(11):1773. doi: 10.3390/cells11111773. Cells. 2022. PMID: 35681468 Free PMC article.
Cited by
-
Delivery technologies for therapeutic targeting of fibronectin in autoimmunity and fibrosis applications.Adv Drug Deliv Rev. 2024 Jun;209:115303. doi: 10.1016/j.addr.2024.115303. Epub 2024 Apr 6. Adv Drug Deliv Rev. 2024. PMID: 38588958 Review.
-
Plant Cellulose as a Substrate for 3D Neural Stem Cell Culture.Bioengineering (Basel). 2023 Nov 13;10(11):1309. doi: 10.3390/bioengineering10111309. Bioengineering (Basel). 2023. PMID: 38002433 Free PMC article.
References
-
- Baron W, Decker L, Colognato H, ffrench-Constant C. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr Biol. 2003;13:151–155. - PubMed
-
- Behar TN. Analysis of fractal dimension of O2A glial cells differentiating in vitro. Methods. 2001;24:331–339. - PubMed
-
- Birch HL. Extracellular matrix and ageing. Subcell Biochem. 2018;90:169–190. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

