Benefits of neutral polysaccharide from rhizomes of Polygonatum sibiricum to intestinal function of aged mice
- PMID: 36204377
- PMCID: PMC9531825
- DOI: 10.3389/fnut.2022.992102
Benefits of neutral polysaccharide from rhizomes of Polygonatum sibiricum to intestinal function of aged mice
Abstract
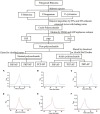
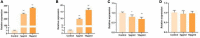
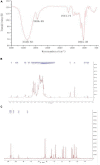
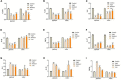
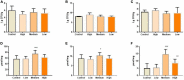
One purified neutral polysaccharide fraction was obtained from the rhizome of Polygonatum sibiricum by DEAE ion exchange and gel chromatography. Structure elucidation was performed by methanolysis, methylation, FT-IR, and NMR. The results indicated that PSP-NP was composed of 1,4-β-D-Gal,1, 4, 6-β-D-Gal, T-α-D-Man,1, 4-α-D-Glc, and T-α-D-Glc with a molecular weight of 43.0 kDa. We supplied this polysaccharide to aged mice and found it is of benefits to intestinal functions, as indicated by better tissue integrity and motility, improved oxidative stress and inflammation, reduced intestinal permeability and serum LPS level, as well as balanced gut microbial composition and short-chain fatty acids production. These results display a novel Polygonatum sibiricum polysaccharide to improve the intestinal function of aged mice, which provides pieces of evidence for its further development and utilization.
Keywords: Polygonatum sibiricum; anti-aging; colon; heteropolysaccharide; jejunum.
Copyright © 2022 Li, Feng, Tao, Paulsen, Huang, Feng, Liu, Yin, Song, Zhao, Liang, Yin, Tang and Zou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

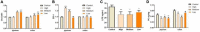

Similar articles
-
Mechanisms of action and applications of Polygonatum sibiricum polysaccharide at the intestinal mucosa barrier: a review.Front Pharmacol. 2024 Aug 19;15:1421607. doi: 10.3389/fphar.2024.1421607. eCollection 2024. Front Pharmacol. 2024. PMID: 39224782 Free PMC article. Review.
-
Chemical elucidation of an arabinogalactan from rhizome of Polygonatum sibiricum with antioxidant activities.Int J Biol Macromol. 2021 Nov 1;190:730-738. doi: 10.1016/j.ijbiomac.2021.09.038. Epub 2021 Sep 11. Int J Biol Macromol. 2021. PMID: 34520778
-
The beneficial effects of Polygonatum sibiricum Red. superfine powder on metabolic hypertensive rats via gut-derived LPS/TLR4 pathway inhibition.Phytomedicine. 2022 Nov;106:154404. doi: 10.1016/j.phymed.2022.154404. Epub 2022 Aug 27. Phytomedicine. 2022. PMID: 36075182
-
Anti-cancer Potential of Polysaccharide Extracted From Polygonatum sibiricum on HepG2 Cells via Cell Cycle Arrest and Apoptosis.Front Nutr. 2022 Jul 4;9:938290. doi: 10.3389/fnut.2022.938290. eCollection 2022. Front Nutr. 2022. PMID: 35903453 Free PMC article.
-
A Review: The Bioactivities and Pharmacological Applications of Polygonatum sibiricum polysaccharides.Molecules. 2018 May 14;23(5):1170. doi: 10.3390/molecules23051170. Molecules. 2018. PMID: 29757991 Free PMC article. Review.
Cited by
-
Antitumor activity of Polygonatum sibiricum polysaccharides.Arch Pharm Res. 2024 Sep;47(8-9):696-708. doi: 10.1007/s12272-024-01511-3. Epub 2024 Jul 26. Arch Pharm Res. 2024. PMID: 39060656 Review.
-
Plant-Based Dietary Fibers and Polysaccharides as Modulators of Gut Microbiota in Intestinal and Lung Inflammation: Current State and Challenges.Nutrients. 2023 Jul 26;15(15):3321. doi: 10.3390/nu15153321. Nutrients. 2023. PMID: 37571257 Free PMC article. Review.
-
Mechanisms of action and applications of Polygonatum sibiricum polysaccharide at the intestinal mucosa barrier: a review.Front Pharmacol. 2024 Aug 19;15:1421607. doi: 10.3389/fphar.2024.1421607. eCollection 2024. Front Pharmacol. 2024. PMID: 39224782 Free PMC article. Review.
-
A New Method of Extracting Polygonatum sibiricum Polysaccharide with Antioxidant Function: Ultrasound-Assisted Extraction-Deep Eutectic Solvents Method.Foods. 2023 Sep 15;12(18):3438. doi: 10.3390/foods12183438. Foods. 2023. PMID: 37761147 Free PMC article.
-
Effects of Solid Fermentation on Polygonatum cyrtonema Polysaccharides: Isolation, Characterization and Bioactivities.Molecules. 2023 Jul 19;28(14):5498. doi: 10.3390/molecules28145498. Molecules. 2023. PMID: 37513370 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

