Cathepsin L-containing exosomes from α-synuclein-activated microglia induce neurotoxicity through the P2X7 receptor
- PMID: 36202834
- PMCID: PMC9537534
- DOI: 10.1038/s41531-022-00394-9
Cathepsin L-containing exosomes from α-synuclein-activated microglia induce neurotoxicity through the P2X7 receptor
Abstract
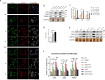
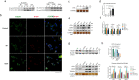
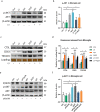
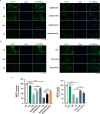
Uncontrolled microglial activation is pivotal to the pathogenesis of Parkinson's disease (PD), which can secrete Cathepsin L (CTSL) to affect the survival of neurons in the PD patients; however, the precise mechanism has yet to be determined. We demonstrated for the first time that CTSL was mostly released by exosomes derived from α-Syn-activated microglia, resulting in neuronal damage and death. The elevation of CTSL activity was blocked by GW4869, suggesting a critical role for exosomes in mediating CTSL release. Furthermore, the P2X7R/PI3K/AKT signalling pathway was identified as the underlying molecular mechanism since specific antagonists of this signalling pathway, P2X7R knockdown and exosome release inhibitors significantly reduced the injury to cultured mouse cortical neurons. Our study suggests that increased extracellular release of CTSL from α-Syn-activated microglia through exosomes amplifies and aggravates of the neurotoxic effect of microglia, implying that CTSL may be involved in a fresh mechanism of PD pathogenesis, and serve as a potential biomarker and a target for PD drug development.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Microglial exosomes facilitate α-synuclein transmission in Parkinson's disease.Brain. 2020 May 1;143(5):1476-1497. doi: 10.1093/brain/awaa090. Brain. 2020. PMID: 32355963 Free PMC article.
-
P2X7 receptor is critical in α-synuclein--mediated microglial NADPH oxidase activation.Neurobiol Aging. 2015 Jul;36(7):2304-2318. doi: 10.1016/j.neurobiolaging.2015.03.015. Epub 2015 Apr 7. Neurobiol Aging. 2015. PMID: 25983062
-
Microglia as modulators of exosomal alpha-synuclein transmission.Cell Death Dis. 2019 Feb 20;10(3):174. doi: 10.1038/s41419-019-1404-9. Cell Death Dis. 2019. PMID: 30787269 Free PMC article.
-
From inflammasome to Parkinson's disease: Does the NLRP3 inflammasome facilitate exosome secretion and exosomal alpha-synuclein transmission in Parkinson's disease?Exp Neurol. 2021 Feb;336:113525. doi: 10.1016/j.expneurol.2020.113525. Epub 2020 Nov 5. Exp Neurol. 2021. PMID: 33161049 Review.
-
Role of exosomes in the pathogenesis of inflammation in Parkinson's disease.Neural Regen Res. 2022 Sep;17(9):1898-1906. doi: 10.4103/1673-5374.335143. Neural Regen Res. 2022. PMID: 35142665 Free PMC article. Review.
Cited by
-
Exploring [11C]CPPC as a CSF1R-targeted PET Imaging Marker for Early Parkinson's Disease Severity.medRxiv [Preprint]. 2024 Feb 13:2023.05.28.23290647. doi: 10.1101/2023.05.28.23290647. medRxiv. 2024. PMID: 37398476 Free PMC article. Preprint.
-
Potential for Therapeutic-Loaded Exosomes to Ameliorate the Pathogenic Effects of α-Synuclein in Parkinson's Disease.Biomedicines. 2023 Apr 17;11(4):1187. doi: 10.3390/biomedicines11041187. Biomedicines. 2023. PMID: 37189807 Free PMC article. Review.
-
Dysregulation of kidney proteases in the pathogenesis of hypertension following unilateral nephrectomy in juvenile mice.Am J Transl Res. 2024 Feb 15;16(2):544-556. doi: 10.62347/HONT9617. eCollection 2024. Am J Transl Res. 2024. PMID: 38463588 Free PMC article.
-
Microglia activation in central nervous system disorders: A review of recent mechanistic investigations and development efforts.Front Neurol. 2023 Mar 7;14:1103416. doi: 10.3389/fneur.2023.1103416. eCollection 2023. Front Neurol. 2023. PMID: 36959826 Free PMC article. Review.
-
Modulation of Microglial Function by ATP-Gated P2X7 Receptors: Studies in Rat, Mice and Human.Cells. 2024 Jan 16;13(2):161. doi: 10.3390/cells13020161. Cells. 2024. PMID: 38247852 Free PMC article. Review.
References
Grants and funding
- 81870920/National Natural Science Foundation of China (National Science Foundation of China)
- 81801262/National Natural Science Foundation of China (National Science Foundation of China)
- 18ZR1428500/Natural Science Foundation of Shanghai (Natural Science Foundation of Shanghai Municipality)
- BK20180991/Natural Science Foundation of Jiangsu Province (Jiangsu Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Miscellaneous

