The effects of fermented vegetable consumption on the composition of the intestinal microbiota and levels of inflammatory markers in women: A pilot and feasibility study
- PMID: 36201455
- PMCID: PMC9536613
- DOI: 10.1371/journal.pone.0275275
The effects of fermented vegetable consumption on the composition of the intestinal microbiota and levels of inflammatory markers in women: A pilot and feasibility study
Erratum in
-
Correction: The effects of fermented vegetable consumption on the composition of the intestinal microbiota and levels of inflammatory markers in women: A pilot and feasibility study.PLoS One. 2024 Jun 24;19(6):e0306219. doi: 10.1371/journal.pone.0306219. eCollection 2024. PLoS One. 2024. PMID: 38913682 Free PMC article.
Abstract
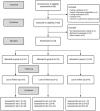
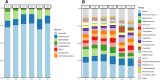
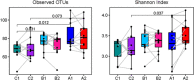
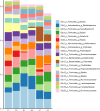
The primary objective of this pilot study was to investigate the feasibility of regular consumption of fermented vegetables for six weeks on markers of inflammation and the composition of the gut microflora in women (clinical trials ID: NTC03407794). Thirty-one women were randomized into one of three groups: 100 g/day of fermented vegetables (group A), 100 g/day pickled vegetables (group B), or no vegetables (group C) for six weeks. Dietary intake was assessed by a food frequency questionnaire and blood and stool samples were provided before and after the intervention for measurement of C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), and lipopolysaccharide binding protein (LBP). Next-generation sequencing of the V4 region of the 16S rRNA gene was performed on the Illumina MiSeq platform. Participants' ages ranged between 18 and 69 years. Both groups A and B had a mean daily consumption of 91g of vegetables for 32 and 36 days, respectively. Serum CRP ranged between 0.9 and 265 ng/mL (SD = 92.4) at baseline, while TNF-α and LBP concentrations ranged between 0 and 9 pg/mL (SD = 2.3), and 7 and 29 μg/mL (SD = 4.4), respectively. There were no significant changes in levels of inflammatory markers among groups. At timepoint 2, group A showed an increase in Faecalibacterium prausnitzii (P = 0.022), a decrease in Ruminococcus torques (P<0.05), and a trend towards greater alpha diversity measured by the Shannon index (P = 0.074). The findings indicate that consumption of ~100 g/day of fermented vegetables for six weeks is feasible and may result in beneficial changes in the composition of the gut microbiota. Future trials should determine whether consumption of fermented vegetables is an effective strategy against gut dysbiosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Role of whole grains versus fruits and vegetables in reducing subclinical inflammation and promoting gastrointestinal health in individuals affected by overweight and obesity: a randomized controlled trial.Nutr J. 2018 Jul 30;17(1):72. doi: 10.1186/s12937-018-0381-7. Nutr J. 2018. PMID: 30060746 Free PMC article. Clinical Trial.
-
Dietary Intervention to Increase Fruit and Vegetable Consumption in Breastfeeding Women: A Pilot Randomized Trial Measuring Inflammatory Markers in Breast Milk.J Acad Nutr Diet. 2018 Dec;118(12):2287-2295. doi: 10.1016/j.jand.2018.06.015. Epub 2018 Sep 10. J Acad Nutr Diet. 2018. PMID: 30213617 Clinical Trial.
-
The Gut Microbiome Is Associated with Circulating Dietary Biomarkers of Fruit and Vegetable Intake in a Multiethnic Cohort.J Acad Nutr Diet. 2022 Jan;122(1):78-98. doi: 10.1016/j.jand.2021.05.023. Epub 2021 Jul 3. J Acad Nutr Diet. 2022. PMID: 34226163 Free PMC article.
-
Consumption of golden berries (Physalis peruviana L.) might reduce biomarkers of oxidative stress and alter gut permeability in men without changing inflammation status or the gut microbiota.Food Res Int. 2022 Dec;162(Pt A):111949. doi: 10.1016/j.foodres.2022.111949. Epub 2022 Sep 17. Food Res Int. 2022. PMID: 36461284
-
Interventions for increasing fruit and vegetable consumption in children aged five years and under.Cochrane Database Syst Rev. 2018 May 17;5(5):CD008552. doi: 10.1002/14651858.CD008552.pub5. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2019 Nov 7;2019(11). doi: 10.1002/14651858.CD008552.pub6 PMID: 29770960 Free PMC article. Updated. Review.
Cited by
-
The effects of fermented vegetables on the gut microbiota for prevention of cardiovascular disease.Gut Microbiome (Camb). 2024 May 9;5:e6. doi: 10.1017/gmb.2024.4. eCollection 2024. Gut Microbiome (Camb). 2024. PMID: 39290661 Free PMC article.
-
Relationships between Habitual Polyphenol Consumption and Gut Microbiota in the INCLD Health Cohort.Nutrients. 2024 Mar 8;16(6):773. doi: 10.3390/nu16060773. Nutrients. 2024. PMID: 38542685 Free PMC article.
-
Diet quality and anxiety: a critical overview with focus on the gut microbiome.Front Nutr. 2024 May 15;11:1346483. doi: 10.3389/fnut.2024.1346483. eCollection 2024. Front Nutr. 2024. PMID: 38812941 Free PMC article.
-
Association between labor epidural analgesia and gut microbiota: A prospective cohort study.Heliyon. 2024 Apr 21;10(9):e29883. doi: 10.1016/j.heliyon.2024.e29883. eCollection 2024 May 15. Heliyon. 2024. PMID: 38699036 Free PMC article.
-
Exploring the Impact of Personalized Nutritional Approaches on Metabolism and Immunity: A Systematic Review of Various Nutrients and Dietary Patterns.Cureus. 2024 Apr 18;16(4):e58553. doi: 10.7759/cureus.58553. eCollection 2024 Apr. Cureus. 2024. PMID: 38765327 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

