The functional importance of VCP to maintaining cellular protein homeostasis
- PMID: 36196920
- PMCID: PMC9704522
- DOI: 10.1042/BST20220648
The functional importance of VCP to maintaining cellular protein homeostasis
Abstract
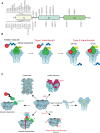
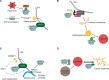
The AAA-ATPase (ATPases associated with diverse cellular activities) valosin-containing protein (VCP), is essential for many cellular pathways including but not limited to endoplasmic reticulum-associated degradation (ERAD), DNA damage responses, and cell cycle regulation. VCP primarily identifies ubiquitylated proteins in these pathways and mediates their unfolding and degradation by the 26S proteasome. This review summarizes recent research on VCP that has uncovered surprising new ways that this ATPase is regulated, new aspects of recognition of substrates and novel pathways and substrates that utilize its activity.
Keywords: autophagy; neurodegeneration; organelles; protein quality control; ubiquitin.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
Valosin containing protein (VCP): initiator, modifier, and potential drug target for neurodegenerative diseases.Mol Neurodegener. 2023 Aug 7;18(1):52. doi: 10.1186/s13024-023-00639-y. Mol Neurodegener. 2023. PMID: 37545006 Free PMC article. Review.
-
The VCP-UBXN1 Complex Mediates Triage of Ubiquitylated Cytosolic Proteins Bound to the BAG6 Complex.Mol Cell Biol. 2018 Jun 14;38(13):e00154-18. doi: 10.1128/MCB.00154-18. Print 2018 Jul 1. Mol Cell Biol. 2018. PMID: 29685906 Free PMC article.
-
A VCP inhibitor substrate trapping approach (VISTA) enables proteomic profiling of endogenous ERAD substrates.Mol Biol Cell. 2018 May 1;29(9):1021-1030. doi: 10.1091/mbc.E17-08-0514. Epub 2018 Mar 22. Mol Biol Cell. 2018. PMID: 29514927 Free PMC article.
-
Interaction between salt-inducible kinase 2 (SIK2) and p97/valosin-containing protein (VCP) regulates endoplasmic reticulum (ER)-associated protein degradation in mammalian cells.J Biol Chem. 2013 Nov 22;288(47):33861-33872. doi: 10.1074/jbc.M113.492199. Epub 2013 Oct 15. J Biol Chem. 2013. PMID: 24129571 Free PMC article.
-
Valosin-Containing Protein (VCP)/p97: A Prognostic Biomarker and Therapeutic Target in Cancer.Int J Mol Sci. 2021 Sep 21;22(18):10177. doi: 10.3390/ijms221810177. Int J Mol Sci. 2021. PMID: 34576340 Free PMC article. Review.
Cited by
-
Anti-Cancer Mechanisms of Diarylpentanoid MS17 (1,5-Bis(2-hydroxyphenyl)-1,4-pentadiene-3-one) in Human Colon Cancer Cells: A Proteomics Approach.Int J Mol Sci. 2024 Mar 20;25(6):3503. doi: 10.3390/ijms25063503. Int J Mol Sci. 2024. PMID: 38542474 Free PMC article.
-
Localized K63 ubiquitin signaling is regulated by VCP/p97 during oxidative stress.bioRxiv [Preprint]. 2024 Jun 21:2024.06.20.598218. doi: 10.1101/2024.06.20.598218. bioRxiv. 2024. PMID: 38948861 Free PMC article. Preprint.
-
Valosin containing protein (VCP): initiator, modifier, and potential drug target for neurodegenerative diseases.Mol Neurodegener. 2023 Aug 7;18(1):52. doi: 10.1186/s13024-023-00639-y. Mol Neurodegener. 2023. PMID: 37545006 Free PMC article. Review.
-
The p97-UBXD8 complex maintains peroxisome abundance by suppressing pexophagy.bioRxiv [Preprint]. 2024 Sep 26:2024.09.24.614749. doi: 10.1101/2024.09.24.614749. bioRxiv. 2024. PMID: 39386596 Free PMC article. Preprint.
-
VCP/p97-associated proteins are binders and debranching enzymes of K48-K63-branched ubiquitin chains.Nat Struct Mol Biol. 2024 Dec;31(12):1872-1887. doi: 10.1038/s41594-024-01354-y. Epub 2024 Jul 8. Nat Struct Mol Biol. 2024. PMID: 38977901 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

