Upregulation of CRABP2 by TET1-mediated DNA hydroxymethylation attenuates mitochondrial apoptosis and promotes oxaliplatin resistance in gastric cancer
- PMID: 36195596
- PMCID: PMC9532395
- DOI: 10.1038/s41419-022-05299-2
Upregulation of CRABP2 by TET1-mediated DNA hydroxymethylation attenuates mitochondrial apoptosis and promotes oxaliplatin resistance in gastric cancer
Abstract
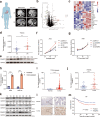
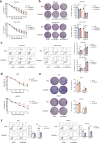
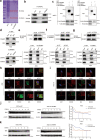
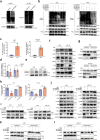
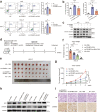
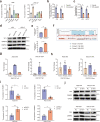
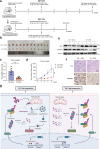
Oxaliplatin is the main chemotherapy drug for gastric cancer (GC), but quite a few patients are resistant to oxaliplatin, which contributes to the poor prognosis of GC patients. There is therefore an urgent need to identify potential targets for reversing chemotherapy resistance in GC patients. In this study, we analyzed the tumor samples of GC patients who received neoadjuvant chemotherapy based on oxaliplatin through quantitative proteomics and identified the potential chemoresistance-related protein cellular retinoic acid binding protein 2 (CRABP2). CRABP2 was significantly upregulated in the tumor tissues of chemoresistant GC patients and was closely related to prognosis. The results of cell function experiments showed that CRABP2 can promote the oxaliplatin resistance of GC cells in vitro. Coimmunoprecipitation and GST pulldown assays showed that CRAPB2 expedited the binding of BAX and PARKIN in GC cells and facilitated the ubiquitination-mediated degradation of BAX. Furthermore, both the in vitro assay and cell-derived xenograft (CDX) in vivo model verified that CRABP2 promoted oxaliplatin resistance by inhibiting BAX-dependent cell apoptosis. Further experiments proved that the abnormally high expression of CRABP2 in oxaliplatin-resistant GC cells was affected by TET1-mediated DNA hydroxymethylation. The patient-derived xenograft (PDX) model suggested that interference with CRABP2 reversed oxaliplatin resistance in GC in vivo. In conclusion, the results of our study show that CRABP2 was a key molecule in oxaliplatin resistance regulation and could be a new target for reversing the chemoresistance of GC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
ATXN2L upregulated by epidermal growth factor promotes gastric cancer cell invasiveness and oxaliplatin resistance.Cell Death Dis. 2019 Feb 20;10(3):173. doi: 10.1038/s41419-019-1362-2. Cell Death Dis. 2019. PMID: 30787271 Free PMC article.
-
Annexin A1 induces oxaliplatin resistance of gastric cancer through autophagy by targeting PI3K/AKT/mTOR.FASEB J. 2023 Mar;37(3):e22790. doi: 10.1096/fj.202200400RR. FASEB J. 2023. PMID: 36786694
-
Up-regulated Wnt1-inducible signaling pathway protein 1 correlates with poor prognosis and drug resistance by reducing DNA repair in gastric cancer.World J Gastroenterol. 2019 Oct 14;25(38):5814-5825. doi: 10.3748/wjg.v25.i38.5814. World J Gastroenterol. 2019. PMID: 31636474 Free PMC article.
-
Modulation of YBX1-mediated PANoptosis inhibition by PPM1B and USP10 confers chemoresistance to oxaliplatin in gastric cancer.Cancer Lett. 2024 Apr 10;587:216712. doi: 10.1016/j.canlet.2024.216712. Epub 2024 Feb 15. Cancer Lett. 2024. PMID: 38364962
-
Cellular Retinoic-Acid Binding Protein 2 in Solid Tumor.Curr Protein Pept Sci. 2020;21(5):507-516. doi: 10.2174/1389203721666200203150721. Curr Protein Pept Sci. 2020. PMID: 32013828 Review.
Cited by
-
Targeting drug-tolerant cells: A promising strategy for overcoming acquired drug resistance in cancer cells.MedComm (2020). 2023 Aug 24;4(5):e342. doi: 10.1002/mco2.342. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37638338 Free PMC article. Review.
-
CRABP2 affects chemotherapy resistance of ovarian cancer by regulating the expression of HIF1α.Cell Death Dis. 2024 Jan 9;15(1):21. doi: 10.1038/s41419-023-06398-4. Cell Death Dis. 2024. PMID: 38195606 Free PMC article.
-
CRABP2 regulates infiltration of cancer-associated fibroblasts and immune response in melanoma.Oncol Res. 2023 Dec 28;32(2):261-272. doi: 10.32604/or.2023.042345. eCollection 2023. Oncol Res. 2023. PMID: 38186580 Free PMC article.
-
Cellular Retinoic Acid Binding Protein 2 (CRABP2), Up-regulated by HPV E6/E7, Leads to Aberrant Activation of the Integrin β1/FAK/ERK Signaling Pathway and Aggravates the Malignant Phenotypes of Cervical Cancer.Biochem Genet. 2024 Aug;62(4):2686-2701. doi: 10.1007/s10528-023-10568-6. Epub 2023 Nov 24. Biochem Genet. 2024. PMID: 38001389
-
Targeting epigenetic regulators to overcome drug resistance in cancers.Signal Transduct Target Ther. 2023 Feb 17;8(1):69. doi: 10.1038/s41392-023-01341-7. Signal Transduct Target Ther. 2023. PMID: 36797239 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

